2006 11 24 CHEM 2302 Assignment 8 (GC) for students
advertisement

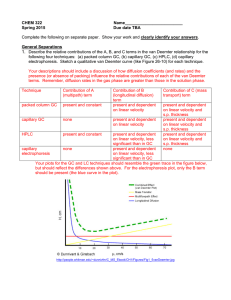
2006 11 24 CHEM 2302* Assignment 8 1. What is the working principle of gas chromatography (GC) in analytical separation? 2. In what circumstances can one use GC instead of HPLC to perform chemical analysis? 3. In the above question, why would the analytical chemist prefer to use GC (instead of HPLC)? What advantages does GC offer? 4. What advantages and disadvantages are there in the use of packed versus open tubular columns in gas chromatography? 5. Bonus question Explain why open tubular columns provide greater resolution than packed columns in gas chromatography. 6. What kind of open-tubular column affords the largest volume of liquid stationary phase for GC analysis? 7. What kind of open-tubular column is the best for GC analysis of vapours from the head space of a beer? 8. What rule(s) can you use to choose a proper liquid stationary phase for a given GC separation? 9. What is the best chromatographic method for the analysis of illicit drugs (e.g., barbital, methadone and cocaine) in a mixture? 10. In GC analysis of a drug mixture, how can you verify that a certain peak on the chromatogram is cocaine, but not barbital or methadone? 11. Draw the molecular structures of PAHs: (a) anthracene, (b) phenanthrene, (c) fluorene, (d) fluoranthene, and (c) benzo[a]pyrene. 12. Bonus question An 11.0 g sediment sample was spiked with 100 L of 380 ppm (or g/mL) D-10 anthracene. After the PAHs were extracted, the solvent was concentrated down to 5.0 mL. The peak produced by D-10 anthracene, as identified by tR, had an area of 184,000. Another peak produced by naphthalene had an area of 1,740,000. Determine the concentration of naphthalene in the original sediment sample (in g/g units). 13. Bonus question Why (and how) do you remove moisture, oxygen and hydrocarbon impurities from the carrier gas used in GC? 14. What is the best possible technique to inject accurately 1 L of sample solution for GC analysis? 15. Describe how the port for split injection to an open tubular column works. 16. What does the silanized glass wool do inside the glass liner of the injection port? 17. When do you need to replace the silanized glass liner inside the injection port? 18. What (range of) temperature is typically found inside the injection port? 19. In GC analysis, what range of column temperature must you use? 20. What will happen to GC peaks if the column temperature is raised? 21. How is the vapour pressure of an organic liquid or compound related to temperature? Can you use this relationship to establish a functional dependence of net retention time on column temperature? 22. A compound is eluted from a GC column at t'r = 15.0 min when the column temperature is 373 K. At 353 K, t'r = 27.1 min. Predict t'r at 363 K. Show all calculation work. 23. What temperature should you use in order to achieve a t'r = 10.0 min? 24. What are the two major objectives of temperature programming in GC? 25. Write a temperature program that is good for the separation of a standard mixture of sixteen PAHs? 26. Bonus question What can the analytical chemist do to eliminate the interference of sample solvent with the GC separation of sample analytes? 27. Is the detector oven set at a lower/higher temperature than the column oven? Typically by how many degrees? 28. What kinds of detectors can be used for GC? 29. What compounds can you determine in air pollution analysis by GC? 30. Blood volatiles analysis of a crime victim has indicated a significant level of methanol. Head space analysis of a beer can is revealing only a low level of methanol. What conclusion (or deduction) can you, as a forensic chemist, draw? 31. Bonus question Gas chromatographic separation of perfume oils is performed on Carbowax 20 M as stationary phase. Three different blends (Vitality, Indulgence, Rejuvenation) obtained from http://www.blackpearlbotanicals.com/ sig_perfume_gift.htm all show a different fingerprinting pattern of GC peaks in the chromatogram than Terra Nova Plumeria Perfume Essence (which is one of the most expensive perfumes on the web market). What compounds can we possibly add to make up the difference in their chemical compositions? 32. What rule(s) can you use to choose a proper liquid stationary phase for a given GC separation? 33. As a stationary phase, what kind of organic compounds is squalene (C30H62) suitable for in GC separation? 34. What would be the bad consequence of using squalene as the liquid stationary phase in gas chromatography for the separation of PAHs? 35. (Diphenyl)x(dimethyl)1-x polysiloxane is a family of common stationary phases used in gas chromatography. What would be the best x value for the separation of ethers, ketones and aldehydes?