lab5 - Stefan Hinote

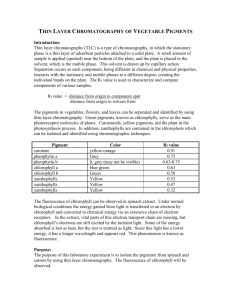
Experimental Procedure A
1) Draw line with pencil 1cm from bottom, 0.6cm side padding
2) Mark 5 1cm intervals on line.
3) Prepare dev chamber, moisten filter with methylene chloride, add solvent to 5mm depth, cap chamber.
4) Spot tlc plate with: fluorene, fluorenol, fluorenone, unknown “C”, standard reference. Use new bone score for each spotting.
5) Place tlc plate in dev chamber, make sure plate does not touch filter paper and spots are higher than solvent depth, cap dev chamber.
6) Remove tlc plate once solvent is within 5mm of end of plate, and mark solvent front.
7) Air-dry tlc plate, view under UV.
8) Place tlc plate in iodine jar, cap, and lightly shake. Mark spots.
9) Measure distance traveled by substances and calculate Rf values.
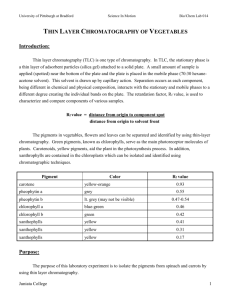
Experimental Procedure B
1) Prepare 4 tlc plates for 3 samples: compound A, compound B, compound A+B.
2) Each dev chamber will have the following solvents: 70% ethyl acetate / 30% hexane
70% hexane / 30% ethyl acetate
Hexane
Acetone
3) Follow steps from part A for preparing tlc plates and dev chambers.
4) Air-dry plates, view under UV, place in iodine chamber.
5) Mark spots, measure distance traveled by substances and calculate Rf values.
Experimental Procedure D
1) Label four 16x100mm test tubes #1-4, prepare 2 dry 5 1/4” pasteur pipets.
2) Add 9.0mL hexane into flask labeled “hexane.”
3) Add 2.0mL acetone into flask labeled “acetone.”
4) Add 2.0mL 70/30 hexane/acetone solution to flask labeled
“70hexane/30acetone.”
5) Stopper each flask.
6) Add 0.3mL of Fluorene-Fluorenone solution into small test tube. Stopper tube.
7) Prepare tlc plates for four spots, use dev chamber with methylene chloride.
8) Add loose plug of glass wool to 5 1/4” pasteur pipet, push down with glass rod, break end of pipet
9) Add 1.25g of Alumina to pipet and tap, clamp in vertical position, place test tube #1 under column
10) With new pasteur pipet add 3mL hexane to column, drain until alumina is moist and hexane level is barely above alumina.
11)Add Fluorene-Fluorenone solution to column with pipet and place test tube
#2 under column. As solution penetrates alumina continue adding hexane until gone. At same time with new pipet add acetone to tip of column to redissolve fluorene solids.
12) Once hexane is used up and solvent level is barely above alumina, add
70/30 hexane/acetone solvent
13) Right before yellow band reaches bottom of column (before glass wool), put test tube #3 under column
14)When eluent is colorless put test tube #4 under column and stop adding solvent.
15)Spot tlc plate with all test tubes and standard reference of fluorenefluorenone.
16)Visualize tlc plate with iodine chamber.
17)Evaporate solvents off in hot bath and run melting point on samples.
Results A
Mixture/Compound Rf
Fluorene 3.4/3.9= 0.87
Fluorenol
Fluorenone
Standard Reference
Unknown “C”
0.9/3.9= 0.23
2.1/3.9= 0.54
1/3.9= 0.2 2.1/3.9= 0.54 3.4/3.9= 0.87
0.9/3.9= 0.23, 2.1/3.9= 0.54
Unknown “C” contained fluorenol and fluorenone based on tlc plate results.
Results B
70% Ethyl Acetate / 30% Hexane
Fluorene 2.75/3.6= 0.76
Fluorenol 2.2/3.6= 0.61
Fluorenone
Unknown “C”
2.5/3.6= 0.69
2.4/3.6= 0.66
Standard Reference 2.5/3.6= 0.69
Poor solvent choice.
70% Hexane / 30% Ethyl Acetate
Fluorene
Fluorenol
Fluorenone
Unknown “C”
Standard Reference
2.9/4.0= 0.73
1.7/4.0= 0.44
2.3/4.0= 0.58
1.6/4.0= 0.40 2.3/4= 0.58
1.6/4.0= 0.40 2.3/4= 0.58 3.8/4= 0.58
Better solvent choice.
Hexane
Fluorene
Fluorenol
Fluorenone
Unknown “C”
Standard Reference
2.1/3.9= 0.54
0.1/3.9= 0.02
0.7/3.9= 0.18
0/3.9= 0 0.2/3.9= 0.05
0/3.9= 0 0.6/3.9= 0.15 2.0/3.9= 0.51
Good solvent choice.
Acetone
Fluorene
Fluorenol
Fluorenone
Unknown “C”
Standard Reference
3.1/3.9= 0.79
2.8/3.9= 0.72
3.0/3.9= 0.77
2.9/3.9= 0.74
2.9/3.9= 0.74
Poor solvent choice.
Results D
Test tube #1
Test tube #2
Test tube #3
Test tube #4
Reference Fluorene-Fluorenone
Test tube #3 product Melting Point
0
0.75
0.43
0.45
0.43 0.75
INSERT TLC PLATE FROM PART D IMAGE
HERE.
83.1C – 83.3C (before correction)
STD Benzoic Acid
MP Correction
Test tube #3 product Melting Point
Test tube #3 melting point after correction
Melting point corresponds with:
+1.55C
(83.1C – 83.3C) +1.55C
84.65C – 84.85C
Fluorenone (82-85C)