TLC Analysis & Mitsunobu Reaction Lab Assignment
advertisement

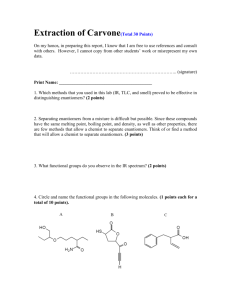
Laboratory Follow-Up Assignment #4 Due: Monday, October 6, 2003 by 5:00 pm Experiment A (TLC of your common analgesic from experiment #3) 1. Turn in pictorial representations of your experimental TLCs and label each of the following: (a) origin (b) solvent front (c) lane identification (d) spot identifications (identify each component) 2. Calculate the Rf values for all spots on your experimental TLC plates and identify your unknown. 3. Do you think that our best solvent system is truly optimal? What might you do in order to improve the experimental conditions in order for us to make better (if not unequivocal) assignments of each standard and the unknown? 4. Explain what would happen if you made the following errors in your TLC experiment: (a) You spotted too much material on your TLC (b) You spotted too little material on your TLC (c) The level of the mobile phase is higher than the applied spot at the (d) You forgot to mark the solvent (e) You used 100% hexane as your mobile phase. plate. plate. origin. front. 5. Complete “Chromatography and Molecular Polarity” (Experiment 1.4 on page 37) from “The Molecular Modeling Workbook for Organic Chemistry” for methyl cyclohexyl sulfide, sulfoxide, and sulfone. (a) Which molecule has the largest dipole moment? Which has the smallest? (b) Which atoms are most positively charged in each molecule? Which are most negatively charged? (c) What effect does increasing oxidation level have on the charge on sulfur (carbon for question #16)? (d) Which molecule is most polar? Which is least polar? (e) Which would you expect to elute first and which one would elute last given a non-polar solvent and a highly polar stationary phase? Experiment B (TLC of the Mitsunobu reaction) 6. Turn in pictorial representations of your experimental TLCs and label each of the following: (a) origin (b) solvent front (c) lane identification (d) spot identifications (identify starting material, product, PPh3, and OPPh3) (e) calculate the Rf values for each spot 7. Looking at the data from question #5, did the reaction go to completion (starting material completely converted into product)? Explain. 8. Which solvent system provided the best mobile phase for visualizing the progress of the reaction? Why is this system the best? Are there others that might work as well? Overall, why is TLC a good method for monitoring the progress of a chemical reaction? 9. Compare triphenylphosphine (one of the starting materials) to triphenylphosphine oxide (one of the products) via Spartan02. Do the same for the starting alcohol and ether product. Using intermolecular forces and the results from modeling to explain why they exhibit different Rf values on the TLC plate. HINT: you must also think about the nature of the plate itself (silica gel -look up in the Aldrich catalog or an MSDS). 10. In what order would you expect to elute 1-butanol, 1-methoxypropane, butanal, and butanoic acid on a silica gel TLC plate developed with dichloromethane? Explain in terms of intermolecular forces. Confirm the ordering by modeling the four compounds using Spartan02. You will need to build and minimize each at the AM1 level before performing the dipole moment, atomic charge, and electrostatic potential map determinations.