File
advertisement

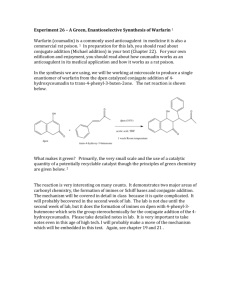
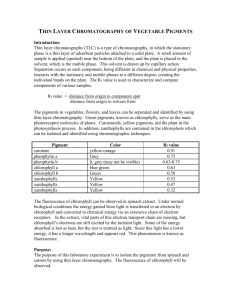
Thin Layer Chromatography - TLC TLC is a simple, quick, and inexpensive procedure that gives the chemist a quick answer as to how many components are in a mixture. TLC is also used to support the identity of a compound in a mixture when the Rf of a compound is compared with the Rf of a known compound (preferably both run on the same TLC plate). A TLC plate is a sheet of glass, metal, or plastic which is coated with a thin layer of a solid adsorbent (usually silica or alumina). A small amount of the mixture to be analyzed is spotted near the bottom of this plate. The TLC plate is then placed in a shallow pool of a solvent in a developing chamber so that only the very bottom of the plate is in the liquid. This liquid, or the eluent, is the mobile phase, and it slowly rises up the TLC plate by capillary action. As the solvent moves past the spot that was applied, an equilibrium is established for each component of the mixture between the molecules of that component which are adsorbed on the solid and the molecules which are in solution. In principle, the components will differ in solubility and in the strength of their adsorption to the adsorbent and some components will be carried farther up the plate than others. When the solvent has reached the top of the plate, the plate is removed from the developing chamber, dried, and the separated components of the mixture are visualized. If the compounds are colored, visualization is straightforward. TLC Adsorbent and Solvent Usually, silica gel plates (SiO2) are used almost exclusively. (Alumina (Al2O3) can also be used as a TLC adsorbent.) In this lab, staining (natural) was used to visualize the components. Acetone was used as the primary solvent. Interactions of the Compound and the Adsorbent The strength with which an organic compound binds to an adsorbent depends on the strength of the following types of interactions: ion-dipole, dipole-dipole, hydrogen bonding, dipole induced dipole, and van der Waals forces. With silica gel, the dominant interactive forces between the adsorbent and the materials to be separated are of the dipole-dipole type. Highly polar molecules interact fairly strongly with the polar Si—O bonds of these adsorbents and will tend to stick or adsorb onto the fine particles of the adsorbent while weakly polar molecules are held less tightly. Weakly polar molecules thus generally tend to move through the adsorbent more rapidly than the polar species. Materials Acetone Baby food jars Dried leaves Microscope slides Pasteur pipets Petroleum ether Silica gel H Stirring rod Preparation of TLC Plates To make the silica gel slurry, acetone is used as the solvent . 1. Mix 20g of silica gel H with 70mL of acetone in a glass bottle large enough to hold two microscope slides (a baby food jar works well). This amount of slurry will coat at least 20 slides. If the slurry becomes thick due to evaporation of the solvent, add a few more mL of acetone and stir again. 2. Stir the mixture well with a glass stirring rod. 3. Place two glass slides back to back with one slightly higher than the other. Dip the slides into the slurry and remove slowly touching the bottom of the slides to the edge of the container to drain off excess slurry. Carefully separate the slides and set them aside to dry. Drying will take only 10 or 20 seconds. Preparation of Leaf Extract 4. Pulverize 2.5g of dried leaves (or material of chocice) with a mortar and pestle. Microwave in 20 sec intervals. Fresh or frozen spinach leaves work well if dried for 48 hours in an oven or incubator at 37° C. If an oven or incubator is not available for drying the leaves, fresh leaves may be macerated with a mortar and pestle. Maceration is more difficult with fresh leaves but the pigment separation, #9 and #10, occurs in a similar manner. 5. Mix the 2.5g of pulverized leaves with 3mL of acetone. Filter this mixture through filter paper into a dark colored, easily stoppered bottle. If fresh leaves are use, mix 10mL of acetone with 2.5g of macerated leaves. Caution: Acetone is a toxic and extremely inflammable substance. Placing Extract on Slide 6. Place a micropipette or pasteur pipet into the leaf extract and allow the solution to move into the pipet by capillary action. 7. Place the pipet on the silica gel about 1mm from the bottom of the slide allowing the solution to run onto the gel. The spot of solution will quickly dry. A second spot should be added and allowed to dry. Continue this procedure until a dark spot is obtained. For demonstration purposes and purely qualitative separations, large micropipets such as pasteur pipets work quite well. For more refined separations, true micropipets should be used. Developing the Slide 8. Place 1.5-2.0mL of chromatographic solution, a mixture of 4.5 parts of petroleum ether and one part acetone, into the chromatojar. The chromatojar should be slightly larger than the microscope slide and should have a tight cover (a baby food jar works well). Again, caution: This solution is toxic and very inflammable. 9. Place the slide in the chromatojar containing the solution and quickly cover. Separation of the pigments will occur in three or four minutes as the solvent moves up the slide. 10. When the solvent front nears the top of the silica gel, remove the slide from the chromatojar and allow to dry. Drying will take about 10 seconds. The chromatojar and solution may be reused many times. 11. Observe the pigments that have separated on the slide. As with paper chromatography, carotenes will be at the top of the slide; lutein, a xanthophyll, appears next as a dark line; the bluegreen color of chlorophyll a will be seen next; and finally near the bottom of the slide a yellow green mixture of chlorophyll b and xanthophylls is seen. leaf pigments color carotenes golden pheophytin olive green chlorophyll a blue green chlorophyll b yellow green lutein yellow xanthophylls yellow