Class Group Activity # 17

CHEMISTRY 212 SPRING 2013 x a
Model 1
Ethane
Energy Diagram for Rotation
Around the C-C bond of Ethane
H
H a
H x
H H
H
H a
H
H a
H
H H a
H x
H H
H x
H x
3.0 Kcal/mole
H x
H a
H
H H
H
H
Conformational Analysis-1
Model 2
Butane
Energy Diagram for Rotation
Around the C
2
-C
3
Bond of Butane
x a
3
3
H
CH
3
CH
3
HCH
3
H
H H
H
CH
3
H
4.5 Kcal/mole
CH
3
H
HCH
3
CH
3
CH
3
H
H
CH
3
H
3
H
5.3 Kcal/mole
H
H
3 3
E
E
2.8 Kcal/mole 3.6 Kcal/mole
0 60 120 180 240 300 360 0.8 kcal/mole
Dihedral Angle Between H
a
& H
x
0 60 120 180 240 300
Dihedral Angle Between CH
3
's
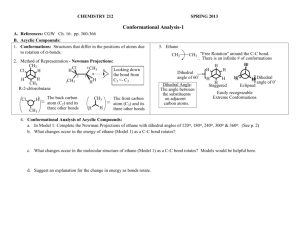
The energy of the molecule varies with rotation around the C-C bond
4. Conformational Analysis of Acyclic Compounds: a.
In Model 1: Complete the Newman Projections of ethane with dihedral angles of 120 o , 180 o , 240 o , 300 o & 360 o . ( See above ) b.
What changes occur in the energy of ethane (Model 1) as a C-C bond rotates?
360
The energy rises and falls in regular cycle (sign wave function) c.
What changes occur in the molecular structure of ethane (Model 1) as a C-C bond rotates? Models would be helpful here.
As the bond rotates, the substituents change position with respect to each other. In a staggered conformation the substituents are as far a part as possible. As the C-C bond rotates the substituents get progressively closer together until they are as close as possible in the eclipsed position.
2 Conformational Analysis-1 d.
Suggest an explanation for the change in energy as bonds rotate.
Rotational Barriers
The energy diagrams indicate that the eclipsed conformations are generally the highest energy conformations and the staggered conformations are the lowest. It seems that bringing substituents closer together raises the energy of a molecule. Thus the higher energy of the eclipsed conformations creates a "rotational energy barrier" which makes bond rotation more difficult. Since this
"rotational energy barrier" exists even in ethane where the substituents are hydrogen atoms, the barrier seems to be at least partly due to e — e repulsion of the electron clouds of the bonds on adjacent carbon atoms as the electron clouds are forced closer together in the eclipsed conformation.
e.
Complete the 3 additional Newman Projections of butane. ( See above .) f.
How does the Energy Diagram of Butane Model 2) compare with that of Ethane (Model 1)?
For ethane all peaks and valleys of the energy curve had the same magnitude. For butane the energy differences between eclipsed and staggered conformations vary as the C-C bond rotates. The differences seem to depend on the relative positions of the two methyl substituents; the energies at 60˚ and 300˚ are the same and those at 120˚ and 240˚ are the same and these pairs of conformations are mirror images of each other with the same relative positions of the methyl groups. g.
Suggest an explanation for the differences between the rotation diagrams for ethane and butane and provide your warrant.
Additional Steric Factors
It appears that some characteristic of methyl groups increases the energy of their interactions with other substituents compared to the same interactions of hydrogen atoms . We explained the energy changes in ethane as changes in e - e repulsion. Can we now extend that e - e repulsion argument to explain the effects seen with methyl groups? Looking at a model of butane compared to that of ethane, we could conclude that the e cloud of a methyl group should occupy a considerably larger volume than that occupied by a hydrogen atom . With this assumption, we could conclude that as groups get larger, their electron clouds are forced closer together in the eclipsed conformation. Thus there is more e - e repulsion as adjacent groups get larger and the energy of the molecule rises higher.
The steric factors even affect some of the staggered conformations. Note that the
"gauche" butane conformations (60° and 300° in the butane diagram) are higher in energy than the "fully staggered" anti conformation (180°)
. This difference in energy is due to the effect of steric interactions of methyl groups “gauche” to each other, compared to interactions of methyl groups that are
“gauche” to only hydrogen atoms.
Detailed Analysis of Relative Effects of Methyl Groups vs. Hydrogen Atoms on Energies
Methyl-Methyl eclipsed Interactions
If we assume that the fully staggered butane and staggered ethane are approximately equal in energy, we can estimate that the highest energy eclipsed conformation for butane, 0° with an eclipsed set of methyl groups, is ~2.3 kcal/mole higher than eclipsed ethane; 5.3 kcal/mole barrier for butane (0˚ vs. 180˚) - 3.0 kcal/mole barrier for ethane (0˚ vs. 60˚).
3 x a
H
H a
H x
H H
H
Ethane
H a
H
H a
H
H H a
H x
H H
H x
H x
3.0 Kcal/mole
H x
H a
H
H H
H
H x a
Conformational Analysis-1
3
3
Butane
H
CH
3
CH
3
H H
H
HCH
3
CH
3
H
H
4.5 Kcal/mole
CH
3
H
HCH
3
CH
3
CH
3
H
H
CH
3
H
3
H
5.3 Kcal/mole
H
H
3 3
E
E
2.8 Kcal/mole 3.6 Kcal/mole
0 60 120 180 240 300 360 0.8 kcal/mole
Dihedral Angle Between H
a
& H
x
0 60 120 180 240 300 360
Dihedral Angle Between CH
3
's
Methyl-Hydrogen eclipsed Interactions
The other eclipsed conformations of butane, where there are two methyl-hydrogen atom eclipsed interactions (120˚ and 240˚) show an energy increase of ~ 0.6 kcal/mol compared with hydrogen-hydrogen eclipsed interactions in ethane [3.6 kcal/mole for butane (120˚ vs. 180˚) – 3 kcal/mole for ethane (0˚ vs. 60˚)].
Methyl-Methyl “Gauche” Interactions
The steric factors even affect some of the staggered conformations. Note that the
"gauche" butane conformations (60° and 300° in the butane diagram), are higher in energy by ~0.8 kcal/mole than the "fully staggered" anti conformation (180°) . This difference in energy is due to the effect of steric interactions of methyl groups “gauche” to each other, compared to interactions of methyl groups that are “gauche” to only hydrogen atoms. Thus, we can estimate that each methyl-methyl “gauche” interaction will raise the energy of a staggered conformation by ~0.8 kcal/mole .
General Estimates
So we can estimate the following relative energy increases a.
A methyl-methyl eclipsed interaction is ~2.3 kcal/mole higher than a hydrogen-hydrogen eclipsed interaction .
b.
A methyl-hydrogen eclipsed interaction is ~0.3 kcal/mole higher than a hydrogen-hydrogen eclipsed interaction; two methylhydrogen eclipsed interactions increase energy by ~0.6 kcal/mole above that for two hydrogen-hydrogen eclipsed interactions or that each, so each interaction has half the effect.
c.
A methyl-methyl “gauche” interaction is ~0.8 kcal/mole higher than a hydrogen-hydrogen “gauche” interaction.