KASH 6 GUIDELINES FOR ABSTRACT SUBMISSION
advertisement

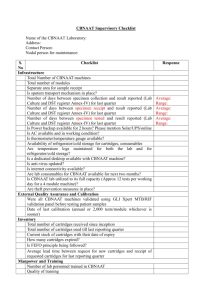
6TH KEMRI ANNUAL SCIENTIFIC & HEALTH (KASH) CONFERENCE 10th to 11th February 2016, KEMRI Headquarters, Nairobi, Kenya 9th Feb 2016 –ASTMH in Kenya Abstract submission: All fields are mandatory Name of corresponding author: Sub-Themes Tel: Tick/Mark one ASTMH in Kenya Pre-conference 9th Feb 2016 Biotechnology Traditional Medicine & Drug Development Infectious and Parasitic Diseases Public Health and Health Systems Non Communicable Diseases Sexual, reproductive, adolescence & Child Health Type of presentation Tick/mark one Oral Poster Paste your abstract in this space, in the format of the provided example (Maximum word count -300 words; provide author affiliations and email address of corresponding author Correlation of GeneXpert MTB/RIF assay quantitative Result and BACTEC MGIT 960system time to detection in sputum samples from MDR suspects in Nyanza province, Western Kenya Sitati R.1, Khayumbi J.1, Shiluli C.2,Okumu A.1, Wandiga S.1, Nduba V.1, Kevin C.3 1 Kenya Medical Research Institute, Centers for Disease Control and Prevention, 2Maseno University, 3 Centers for Disease Control and Prevention, Atlanta, GA, USA Email: rsxxxx@kemricdc.org Background: Approximately 9.4 million incident cases of Tuberculosis (TB) are reported annually. Early detection of resistance to primary TB drugs is essential for treatment and control of MDR tuberculosis. Xpert MTB/RIF assay, an automated real-time PCR based assay and the BACTEC MGIT 960 system®, a liquid culture system, are both recommended for detection of M. Tuberculosis in high burden TB countries. There is limited data on comparison of the two methods. We compared the Xpert MTB/RIF quantitative results and time to detection of M. Tuberculosis (MTB) by MGIT BACTEC 960. Methods: sputum samples from MDR suspects from selected health facilities in Nyanza were collected and received in the KEMRI/CDC tuberculosis laboratory. They were analyzed by both the Xpert MTB/RIF assay and processed for MGIT 960 system. Nalc-NaOH method was used to process these sputum samples for the MGIT 960 cultures and on incubation, time to positivity observed. The Xpert MTB/RIF quantitative results and the positive time to detection by BACTEC MGIT 960 system®, were analyzed. Results:162 samples were MTB positive by the Xpert/MTB/RIF assay, and the MTB DNA quantified as Very high; 61 (38%), Medium,78 (48%), Low18 (11%) and Very low5 (3%). By the Bactec 960 system, these same samples were flagged positive on average in 6 days, 10 days, 14 days, and 17 days respectively. Conclusion: Results from this study indicate a proportional increase in time to detection of MTB by the BACTEC 960 system as the bacterial load decreases as measured by the Xpert/MTB/RIF assay. 6TH KASH CONFERENCE: GUIDELINES FOR AUTHORS To help you with your abstract submission, kindly note the following guidelines All abstracts must be submitted in English language. Abstracts can only be submitted on line via the template provided on the KEMRI web site or email at kash@kemri.org The abstract text should not exceed 300 words, use the format provided in the table. Tables and graphs may be included as attachments. It is the author’s responsibility to submit a correct abstract. Any errors in spelling, grammar or scientific facts will be reproduced as typed by the author. Abstracts must not have been published or submitted for presentation to any other national or international meeting. Choosing Abstract Sub-themes Categories The abstract sub-themes categories are the general heading under which your abstract will be reviewed and later published in the conference printed matters if accepted. Please choose the sub-themes which best describes the subject of your abstract. Notification of Acceptance or Rejection Notification of acceptance or rejection will be sent to the corresponding author by the 15th January 2016. Please note that only the corresponding author will receive mail concerning the abstract and is responsible for informing all co-authors of the status of their abstract. Authors whose abstracts have been accepted will receive instructions for the presentation of their abstract. Abstract Blinding and Review All submitted abstracts will go through a blind peer-review process carried out by an independent reviewing committee. The Scientific Sub-committee will post reviewer’s comments to the corresponding authors through emails. 6th KASH Conference Abstract Policy An abstract cannot be submitted if it has been previously published or presented, unless there are major updates in the data, and may not be presented elsewhere after acceptance for KASH 6. If preliminary or partial data has been published, the author is required to indicate the details of the conference, meeting or journal. However, for the 9th Feb 2016, “ASTMH In Kenya” pre-conference, young researchers may present what they already submitted to the 2015 ASTMH, for local audience. 6th KASH Conference Publication All the accepted abstracts will be published in a special edition of the peer-reviewed African Journal for Health Sciences. If you have any questions regarding your abstract submission, please contact the abstract support team at kash@kemri.org