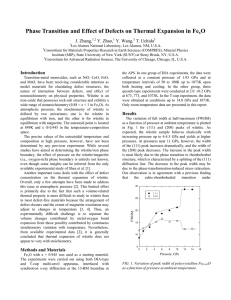
Dielectric constant of the solvent allows control over the growth and

Dielectric constant of the solvent allows control over the growth and surface functionalization of nanocrystalline magnetite.
Srividhya J. Iyengar, Swapankumar Ghosh *
Advanced Clay & Traditional Ceramics Division, CSIR-Central Glass & Ceramics Research
Institute, Kolkata-700032, India.
E-mail: srividhyaji@gmail.com; swapankumar.ghosh2@mail.dcu.ie; Fax:+91 33 24730957; Tel: +91-
33-23223546
Abstract
Highly crystalline magnetite (Fe
3
O
4
) nanoparticles in the size range of 7.8
–13.5 nm were synthesized by homogeneous precipitation in different alcohol/water (1:1) mixed solvents at different temperatures and subsequently surface treated with aminopropyltrimethoxysilane/ tetramethylammonium hydroxide to produce stable ferrofluids. Irrespective of type of alcohol used, the fabricated Fe
3
O
4
exhibit cubic fcc inverse spinal structures. FTIR spectra reveal the binding of coating materials on the surface of NPs. In EG/water solvent, the resultant particles were extremely small of the order 7.42 nm compared to the same of size 13 nm in water due to the changes in electrostatic interactions and nucleation rates with the varying dielectric constants of mixed solvents. The size of the magnetite nanocrystals synthesized was found to decrease with decrease in
of the solvent, especially in the case of mono-ols.
Nanocrystals showed a (311) growth, with highest magnetic susceptibility of ~70 emu.g
−1
. Maghemite content as impurity phase was estimated at ~20% in the powder specimens from XPS and Rietveld analysis. Surface treatment mediated a phase transfer of the magnetite nanocrystals with exposed different crystallographic facets from a hydrophobic to hydrophilic state with potentials applications in imaging and drug delivery in biotechnology, selective catalysis and as ferrofluids.
Key-words : Fe
3
O
4
, nanocrystals, Dielectric constant, Monodisperse, Coprecipitation, Magnetic susceptibility