Intraosseus Stem Cell Transplantation Extends the Survival

Title: Intraosseus injection of the Donor-derived Bone Marrow Stem and Progenitor Cells Increases
Donor-Specific Chimerism and Extends Composite Tissue Allograft Survival
Authors: Selahattin Ozmen, MD, Maciej Zielinski, MD, Kagan Ozer, MD, Dariusz Izycki, MD,PhD,
Maria Siemionow, M.D,PhD
Introduction: Each year, more than 7 million Americans require composite tissue coverage for various types of reconstructive procedures. This is more than double the number of solid organs needed annually
(heart, liver pancreas, kidney). However, there are some obstacles precluding routine use of the composite tissue allograft (CTA) transplants. This includes high immunogenicity of CTAs when compared with solid organ transplants, and is related to multiple tissue transplants including highly immunogenic skin component. Moreover, current CTA transplantation protocols require use of high dose, life-long immunosuppressive protocols, making routine use of CTA transplants questionable.
Methods used for prevention of allotransplant rejection include donor’s and recipient’s HLA antigens matching, specific or non-specific immunosuppression, and induction of donor specific tolerance. The ultimate goal for CTA transplantation is the induction of the donor specific tolerance without need for chronic immunosuppression. Recent approaches include transplantation of donor derived hematopoietic stem cells for tolerance induction.
1-3
Transplanted donor’s stem cells engraft into the recipient’s bone marrow and lymphoid organs, repopulate, and create multilineage donor specific chimerism which can lead to donor specific tolerance.
4
The limitations of bone marrow transplantation (BMT) include toxicity of the myeloablative conditioning protocols, graft versus host disease, and failure of the bone marrow cell engraftment.
Purpose: Based on the idea to create hematopoietic chimerism and to extend allograft survival, in this study we evaluated the impact of the intraosseous allotransplantation of the donor specific stem and early progenitor cells on the level of donor specific chimerism and survival rate of the rat hind limb allograft transplants without immunosuppressive treatment protocols.
Method: Eighteen rat hind limb transplantations were performed in three groups (Fig 1). Isograft transplantations were done between Lewis (LEW, RT1
1
) rats and allograft transplantations were performed from Lewis-Brown-Norway (LBN, RT1 n+1
,F1) to Lewis rats across MHC mismatched barrier
(Fig 2a). Isografts and allografts rejection controls (groups I and II) received no treatment. In-group III,
0.8 - 1.2x106 /50
l of CD90+ hematopoietic stem and progenitor cells were injected into the recipient’s opposite tibial bone marrow cavity just before limb transplantation ( Fig. 1 ).
Donor bone marrow was harvested from the donor’s tibia and CD 90 + cells were isolated. Donor bone marrow stem and progenitor cells separation was performed by targeting stem and progenitor cells with mouse anti-rat FITC - conjugated CD90 mAb (Clone - OX-7; BD, San Jose, CA) and tagging with goat anti FITC magnetic-beads conjugated, followed by isolation on the MidiMACS LS+ columns.
Separation and purification of the CD90 + stem and early progenitor cells was confirmed with flow cytometry (efficacy >95%)
Limb Transplantation Procedure: Under sodium-pentobarbital (50 mg/kg, IP, Nembutal
®
Abbott
Laboratories, Chicago, IL) anesthesia recipient’s and donor’s right hind limbs were amputated at midfemoral level. Donor hind limb was irrigated with cold heparinized saline. Following rigid fixation of the donor and recipient’s femoral bone stumps muscle layers were repaired. Femoral and sciatic nerves were repaired and femoral vessels anastomoses was performed under microscopic magnification. Skin closure completed transplantation procedure, which took 1.5 hours with the ischemia time of 45 minutes.
Intraosseous transplantation of the donor derived CD90
+
cells ( Fig. 1 ): For intraosseous BM transfer, a hole was created on the tibial diaphysis. Bone marrow content of recipient bone was aspirated by vacuum created with a syringe. Next, preoperatively prepared CD90+ hematopoietic and progenitor cells solution was injected into the tibial bone marrow cavity through the hole. For the prevention of the leakage, the tibial hole was occluded by bone wax. Skin was sutured with absorbable suture material.
All allograft recipients’s skin was evaluated daily for any signs of rejection such as; erythema, edema, hair loss, scaling, desquamation and necrosis.
Fig. 1: Intraosseous injection of the CD90
+ hematopoietic stem and progenitor cells.
Results: Transplants in Isograft Control Group survived indefinitely. In Allograft Control Group
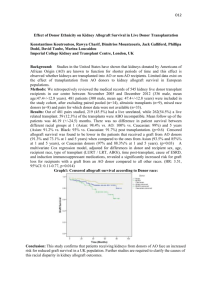
(without stem cell injection), transplants were rejected within 6 to 7 days. Following intraosseous stem cell injection (Group III) limb survival was extended up to 15 days post-transplant ( Table 1, Fig 2 ).
Flow Cytometric assessment of the donor specific chimerism at day 14 post-transplant showed 3.4%
CD4+/RT1n double positive in the stem cell treatment group, whereas the level of lymphoid chimerism in allograft rejection group was significantly lower (0.6%) ( Fig. 3 ).
Table 1. Transplantation groups, treatment protocols and survivals of the transplants.
No. GROUP N PROCEDURE THERAPY Survival time
I Isograft Control 6 Limb Isotransplantation No Indefinite
II Allograft Control 6 Limb Allotransplantation No 7 days
III Allograft Treatment 6 Limb Allotransplantation 0.8-1.2x10
6
CD90+ cells
Up to 15 days
P<0.05
Conclusion: In this study, we introduced a novel method of transplantation of the stem and early progenitor cells of the donor origin into the recipient’s bone marrow cavity. This technique of intraosseus injection of donor stem cells resulted in the significant extension of allograft survival. The technique is minimally invasive, does not require recipient conditioning, and thus, may have a potential for clinical applications. Further studies combining immunomodulatory therapies and stem cell transfer are in progress.
Limb Allograft Survival
100
90
80
70
60
50
40
30
20
10
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30
Day
Isograft Allograft + (CD90+) cell Allograft
Fig 2: Survival chart of the transplants. Isograft transplants survived indefinitely. Allograft transplants without intraosseous CD90+ cells injection were rejected within 6-7 days post-transplant. In-group III
RT1 l+n /CD4+
3.4%
RT1 l+n /CD4+
0.6%
CD4+ - PE CD4+ - PE
RT1l+n – FITC
Allograft & CD90+ cells Allograft - control
Fig. 3: Flow Cytometric assessment of the donor specific chimerism at day 14 post-transplant showed
3.4% CD4+/RT1n double positive in-group III, however the level of lymphoid chimerism in-group II was significantly lower (0.6%).
References
1 Bagley J, Tian C, Sachs DH, Iacomini J: Induction of T-cell tolerance to an MHC class I alloantigen by gene therapy. Blood 99(12):4394-9, 2002
2 Hashimoto N, Narumi S, Itabashi Y, Hakamada K, Sasaki M: Efficacy of donor splenocytes mixed with bone marrow cells for induction of tolerance in sublethally irradiated mice. Transpl
Immunol 10(1):37-41, 2002
3 Yin D, Ma L, Zeng H, Shen J, Chong AS: Allograft tolerance induced by intact active bone cotransplantation and anti-CD40L monoclonal antibody therapy. Transplantation 74(3):345-54,
2002
4 Srour EF, Jetmore A, Wolber FM: Homing, cell cycle kinetics and fate of transplanted hematopoietic stem cells. Leukemia 15(11):1681-4, 2001