ABO incompatible living donor renal transplantation in West London
advertisement

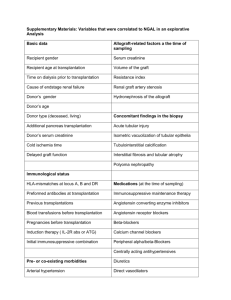
P299 ABO INCOMPATIBLE LIVING DONOR RENAL TRANSPLANTATION IN WEST LONDON Galliford, J, Lawrence, C, Charif, R, Chan, K, Willicombe, M, Lee, J, Roufosse, C, Cook, T, Warrens, A, Cairns, T, McLean, A, Hakim, N, Papalois, V, Taube, D West London Renal and Transplant Centre, Hammersmith Hospital, London INTRODUCTION: ABO incompatible living donor [ABOiLD] transplantation is a successful and accepted form of treatment for patients with renal failure, performed by an increasing number of transplant centres in the UK. The purpose of this study is to report our experience of ABOiLD transplantation, which is currently the largest programme in Europe. METHODS: Since 2004, 52 patients [30m, 22f; mean age 48.6±11.2 years] entered the programme. Patients have received plasma exchange after Rituximab [1g] or Campath [30mg] to achieve a pre-transplantation anti-blood group IgG antibody titre of ≤1/4. Oral immunosuppression has been Tacrolimus based and steroid sparing. Mycophenolate Mofetil was used pre-Campath era. Comparison is made with 355 ABO compatible living donor [ABOcLD] transplants performed over the same time period. RESULTS: Six patients [11.5%] did not reach the required IgG titre for transplantation. 46/52 patients [25m, 21f; mean age 50.54±12.45 years] were transplanted and have been followed for a mean of 24.5±17.8 months [range 0.5-78 months]. This represents 11.8% of living donor transplantation during the same period. 16/46 [34.8%] were pre-emptive transplants, 8/46 [17.3%] regrafts, 36/46 [78.2%] unrelated donors and the mean mismatch was 3.6±1.6. Patient survival is 100%. Allograft survival at 1, 2 and 4 years is 95.6%, 84.0% and 84.0%. There have been 4 lost allografts which were due technical failure [n=2], 1 acute antibody mediated rejection, 1 for a combination of non-adherence/ recurrent disease/ rejection and 1 after immunosuppression was withdrawn in severe sepsis. Allograft function [eGFR; MDRD] at 1, 2 and 4 years is excellent and stable at 49.7±10.7, 51.1.1±13.5 and 51.0±14.5 mls/min/1.73m2 and compare favourably to ABOcLD transplants at 54.6±16.2 [p=0.10], 51.7±14.9 [p=0.48] and 50.9±13.4 [p=0.98] mls/min/1.73m2. AR was significantly more frequent in ABOiLD patients [22/46 (45.6%)] than in ABOcLD patients [66/355 (18.6%); p<0.0001]. CONCLUSION: This study shows that ABOiLD transplantation is successful and has similarly excellent medium term outcomes as our ABOcLD programme. Our results are comparable to international competitors. It is a simple and safe way of increasing living donor transplantation. Although there is a higher rate of rejection, there has been no impact on allograft function as yet.