Chapter 4 - People.vcu.edu
advertisement

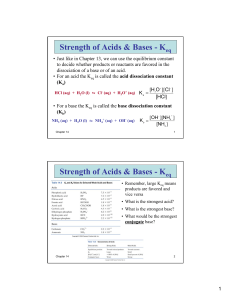
Chapter 4 Halogenation Addition of halogen to an alkane o Only works on sp3-hybridized carbons Works best with chlorine and bromine o Iodine is too slow o Fluorine blows up (too fast) Goes through radical intermediate, so putting halogen on more substituted carbon is better o Bromine is way more selective than chlorine Initiation o Homolytic cleavage of X2 2 o One molecule splits into two radicals Propagation o Start with one molecule and one radical; end up with one molecule and one radical! . 1) . 2) Termination o Any two radicals stick together . . . + HCl Reactive Intermediates Carbocations o Carbon with three bonds and no lone pairs o Trigonal planar geometry o sp2-hybridized o empty p-orbital o stability - 3°>2°>1°> methyl o electron-deficient o only intermediate which can undergo rearrangement hydride shift 1° carbocation with 3° position next door We’ll see a lot of this later in the course. It’s a good idea to think “carbocations can rearrange” every time you see a carbocation intermediate for the rest of your life! Radicals o Carbon with three bonds and one unpaired electron o Trigonal planar geometry o sp2-hybridized o one electron in the p-orbital o stability - 3°>2°>1°> methyl o electron-deficient Carbanions o Carbon with three bonds and a lone pair o sp3-hybridized o Tetrahedral geometry o Stability- methyl>1°>2°>3° o Strong bases/nucleophiles Carbenes o Carbon with two bonds and one lone pair o Neutral o Rare o sp2-hybridized o empty p-orbital o can be electrophile or nucleophile Radicals, carbocations, and carbanions are stabilized by resonance o One away from a double bond is called “allylic” Being directly on the double bond is particularly unstable and this position is called “vinyl” o One away from an aromatic ring is called “benzylic” Being directly on the aromatic ring is particularly unstable and this position is called “aryl” The rest of chapter 4 that you should know from Gen Chem Equilibrium Constant o Keq= o If Keq> 1, then more products than reactants o If 0<Keq<1, then more reactants than products o He may ask you to propose a Keq for a reaction based on some information. If he tells you a reaction is highly exergonic, then your Keq should be really large. Think 103 or higher If he tells you a reaction is highly endergonic, then your Keq should be really small. Think 10-3 or smaller. If he wants one of these extremes and you give a value like 1.5 or .7, he won’t give full credit. ∆G=∆H-T∆S o If ∆G<0, then spontaneous This is called exergonic. o If ∆G=0, then at equilibrium o If ∆G>0, then non-spontaneous This is called endergonic. Bond-Dissociation Energies o If ∆H<0, then the reaction is exothermic. o If ∆>0, then the reaction is endothermic. o ∆H of a reaction can be estimated by ∆H=bonds broken-bonds formed Kinetics o For A + B → C + D o rate=k[A]m[B]n o The overall reaction order is m + n o A is of the mth order and B is of the nth order Activation Energy o The difference in energy from the reactants to the highest-energy transition state Transition States o Chemical species which exist fleetingly o Structure or species when bonds are breaking and/or forming all at once o Occur at the peaks of a reaction coordinate diagram Intermediates o Chemical species which occur at the troughs of a reaction coordinate diagram o Exist long enough to be isolated This means that he will not use the word fleetingly when describing them. Reaction coordinate diagrams o The number of humps is the number of steps in a reaction. o The valleys are intermediates. o At the top of each hump is a transition state. o The highest hump is the rate-determining step.