Hw Worksheet: Atomic Structure & The Periodic
advertisement

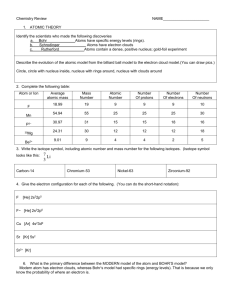
Name___________________ Hw: Atomic Structure and Table of the Elements Use the periodic Table of the Elements to fill in the chart below: Element Atomic number Number protons Number electrons Number neutrons Atomic Mass Lithium Oxygen Argon Mercury Lead Carbon Iron Hydrogen Potassium Sodium Chlorine Gold Name five elements that are gasses at room temperature. Name five elements that are solids at room temperature. Name two elements that are liquids at room temperature. Name five elements that float in water. Name five elements that sink in water. Mass Number In the space below, diagram the electron configuration of a hydrogen atom. In the space below, diagram the electron configuration of a silicon atom. In the space below, diagram the electron configuration of a potassium atom. Below are electron configurations of two atoms. Tell the names of the elements, the atomic numbers, and the mass numbers for each. What are the atomic masses and mass numbers of the following elements? magnesium lithium oxygen potassium iron Write the symbols of the following elements: manganese mercury nickel nitrogen hydrogen calcium copper potassium sodium sulfur What is an isotope? Give an example. What is an ion? Give an example. Complete the following 1. Use your Periodic Table of the Elements to complete the chart below. Make note of the charge to determine whether or not it represents an ion. Symbol Charge K + Protons Electrons +2 Cl 12 18 18 F Atomic number 16 neutral 10 2. An atom has 11 protons and 12 neutrons and 10 electrons A. What is its mass number? B. What is its atomic number? C. What is its charge? D. How many electrons are in the outer most shell? E. What is the symbol for this element? 2. Indicate whether the following elements are solid, liquid or gas at room temperature (20 °C) iodine, magnesium, nitrogen, carbon, mercury, oxygen 3. Sketch a temperature vs. time graph that shows all the phase changes for the following elements: Iron carbon bromine