Penn Freshman Lab
advertisement

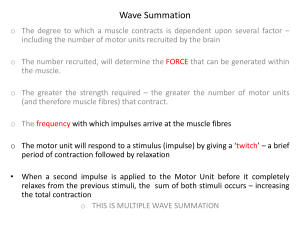
Introduction to Bioengineering Skeletal Muscle Physiology – Recruitment and Fatigue I. OBJECTIVES 1. To understand skeletal muscle and electromyography. 2. To acquire and analyze data with BIOPAC system. 3. To compare the maximum clench force and fatigue time of different subjects. 4. To study motor unit recruitment with increased power of muscle contraction. 5. To study the force produced by contraction, EMG and integrated EMG during fatigue. II. BACKGROUND The introduction sections from Lessons 1 and 2 of BIOPAC Student Lab V3.0 manual are provided below to introduce electromyography. The human body contains three kinds of muscle tissue and each performs specific tasks to maintain homeostasis: Cardiac muscle, Smooth muscle, and Skeletal muscle. Cardiac muscle is found only in the heart. When it contracts, blood circulates, delivering nutrients to cells and removing waste. Smooth muscle is located in the walls of hollow organs, such as the intestines, blood vessels or lungs. Contraction of smooth muscle changes the internal diameter of hollow organs, and is thereby used to regulate the passage of material through the digestive tract, control blood pressure and flow, or regulate airflow during the respiratory cycle. Skeletal muscle derives its name from the fact that it is usually attached to the skeleton. Contraction of skeletal muscle moves one part of the body with respect to another part, as in flexing the forearm. Contraction of several skeletal muscles in a coordinated manner moves the entire body in its environment, as in walking or swimming. The primary function of muscle, regardless of the kind, is to convert chemical energy to mechanical work, and in so doing, the muscle shortens or contracts. Human skeletal muscle consists of hundreds of individual cylindrically shaped cells (called fibers) bound together by connective tissue. In the body, skeletal muscles are stimulated to contract by somatic motor nerves that carry signals in the form of nerve 1 impulses from the brain or spinal cord to the skeletal muscles (Fig. 1.1). Axons (or nerve fibers) are long cylindrical extensions of the neurons. Axons leave the spinal cord via spinal nerves and the brain via cranial nerves, and are distributed to appropriate skeletal muscles in the form of a peripheral nerve, which is a cable-like collection of individual nerve fibers. Upon reaching the muscle, each nerve fiber branches and innervates several individual muscle fibers. Figure 1 Example of Motor Units Although a single motor neuron can innervate several muscle fibers, each muscle fiber is innervated by only one motor neuron. The combination of a single motor neuron and all of the muscle fibers it controls is called a motor unit (Figure 1). When a somatic motor neuron is activated, all of the muscle fibers it innervates respond to the neuron’s impulses by generating their own electrical signals that lead to contraction of the activated muscle fibers. Note: The size of the motor unit arrangement of a skeletal muscle (e.g., 1:10, 1:50, or 1:3000) is determined by its function (flexion, extension, etc.) and the location in the body. The smaller the size of a muscle’s motor units, the greater the number of neurons needed for control of the muscle, and the greater the degree of control the brain has over the extent of shortening. For example, muscles which move the fingers have very small motor units to allow for precise control, as when operating a computer keyboard. Muscles that maintain posture of the spine have very large motor units, since precise control over the extent of shortening is not necessary. Physiologically, the degree of skeletal muscle contraction is controlled by: 1. Activating a desired number of motor units within the muscle, and 2. Controlling the frequency of motor neuron impulses in each motor unit. 2 When an increase in the strength of a muscle’s contraction is necessary to perform a task, the brain increases the number of simultaneously active motor units within the muscle. This process is known as motor unit recruitment. Resting skeletal muscles in vivo exhibit a phenomenon knows as tonus, a constant state of slight tension that serves to maintain the muscle in a state of readiness. Tonus is due to alternate periodic activation of a small number of motor units within the muscle by motor centers in the brain and spinal cord. Smooth controlled movements of the body (such as walking, swimming or jogging) are produced by graded contractions of skeletal muscle. Grading means changing the strength of muscle contraction or the extent of shortening in proportion to the load placed on the muscle. Skeletal muscles are thus able to react to different loads accordingly. For example, the effort of muscles used in walking on level ground is less than the effort those same muscles expend in climbing stairs. When a motor unit is activated, the component muscle fibers generate and conduct their own electrical impulses that ultimately result in contraction of the fibers. Although the electrical impulse generated and conducted by each fiber is very weak (less than 100 μvolts), many fibers conducting simultaneously induce voltage differences in the overlying skin that are large enough to be detected by a pair of surface electrodes. The detection, amplification, and recording of changes in skin voltage produced by underlying skeletal muscle contraction is called electromyography. The recording thus obtained is called an electromyogram (EMG). Mechanical work, in the physical sense, refers to the application of a force which results in the movement of an object. Skeletal muscle performs mechanical work when the muscle contracts and an object is moved, as in lifting a weight. To lift a weight, your muscles must exert a force great enough to overcome the weight. If you exert less force, then the weight does not move (Figure 2). Figure 2. Lifting a weight with a force great enough to overcome the weight or with a force not great enough to overcome the weight. Physiologically, skeletal muscle is stimulated to contract when the brain or spinal cord activates motor units of the muscle. 3 Note: Motor units are defined as a motor neuron and all of the muscle fibers that the motor neuron innervates. An action potential (AP) in a human motor always causes an action potential in all of the muscle fibers of the motor unit. As a matter of fact, humans generally do not send just one AP at a time down a motor neuron. Instead, a train of APs is sent – enough to induce tetany (the sustained fusion of individual muscle twitches) in the muscle fibers of the motor unit. Most human skeletal muscles are composed of hundreds of motor units (see Figure 1 again). When a skeletal muscle is called on to perform mechanical work, the number of motor units in the muscle activated by the brain is proportional to the amount of work to be done by the muscle: the greater the amount of work to be done, the greater the number of motor units activated. Thus, more motor units are simultaneously active when a skeletal muscle lifts 20 kilograms than when the same muscle lifts 5 kilograms. The brain determines the number of active motor units required for a muscle to perform a given task by utilizing sensory information from stretch receptors in the muscle and associated tendons and joint capsules. For example, when lifting a bucket of water from the ground, the brain first activates several motor units in the requisite skeletal muscles. If sensory information returning from the muscles indicates the muscles are contracting but not developing adequate power to lift the bucket, the brain activates additional motor units until the sensory information indicates the bucket is being lifted. The sequential activation of motor units to perform a designated task is called motor unit recruitment. Once you have lifted a light object, the brain recruits approximately the same number of motor units to keep the object up, but cycles between different motor units. The muscle fibers consume stored energy available in the muscle, and generate a force by contracting. As the muscle fibers deplete this fuel source, more energy must be created in order to continue contracting. By recruiting different motor units, motor units can relax and replenish their fuel sources. Skeletal muscles performing acute maximum work or chronic sub-maximum work of a repetitive nature will eventually fatigue. Fatigue is defined as a decrease in the muscles’ ability to generate force. Fatigue is caused by a reversible depletion of the muscle’s fuel supply. If the muscle uses its energy sources faster than it can be generated by cellular metabolism, fatigue occurs. During contraction, skeletal muscle cells convert chemical energy into thermal and mechanical energy, and, in the process, produce chemical waste products. Normally the waste products are removed from the muscle by the circulatory system as the blood brings nutrients to the muscle for energy transformation. If certain waste products (metabolites) are not removed at an adequate rate, they will accumulate and chemically interfere with the contractile process, thereby hastening the onset of fatigue. Some accumulated waste products also stimulate pain receptors in surrounding connective tissue and induce cramping of skeletal muscle, a general sign of inadequate blood flow to the muscle. 4 Power is defined as the amount of work done per unit of time. Dynamometry means the measurement of power (dyno = power, meter = measure), and the graphic record derived from the use of a dynamometer is called a dynagram. III. MATERIALS In this lesson, you will examine motor unit recruitment and skeletal muscle fatigue by combining electromyography with dynamometry. BIOPAC SS25 Hand Dynamometer BIOPAC SS2L Electrode Lead Set BIOPAC disposable electrodes, 3 per subject BIOPAC MP35 acquisition unit 5 IV. EXPERIMENTAL METHODS The experimental methods are presented in a two-column format. The left column gives a brief explanation of each step. The right column contains more detailed information about the steps or concepts in the Fast Track column. A. Set-up FAST TRACK STEPS Detailed Explanation of Steps 1. Attach three electrodes to the dominant forearm of the subject on the Brachioradialis muscle. Use the dominant forearm of the subject. Generally, if the subject is right-handed, select the right forearm or if left-handed, select the left forearm. You will monitor the Brachioradialis muscle of the forearm. Please refer to the muscle diagram in the lab for proper electrode placement and attach the three electrodes as shown in Fig. 3. Figure 3 2. Attach the electrode lead set to the electrodes, following the diagram shown in Figure 4. Each of the pinch connectors on the end of the electrode cable needs to be attached to a specific electrode. The electrode cables are a different color, and you should follow Fig. 4 to ensure that you connect each cable to the proper electrode. The pinch connectors work like a small clothespin, but will only latch onto the nipple of the electrode from one side of the connector. CONTINUED ON NEXT PAGE 6 3. Measure the arm circumference near the white lead. Figure 4 Also measure the arm circumference near the white lead for each subject. END OF SET-UP 7 B. Recording Lesson Data FAST TRACK RECORDING Detailed Explanation for Recording Lesson Data 1. Prepare for the recording. You will record two experiments. a. Experiment 1 records Motor Unit Recruitment. b. Experiments 2 and 3 record Fatigue. In order to work efficiently, read this entire section before beginning. EXPERIMENT 1 2. Following instructions are for PCs in M204 3. Check for Bioengineering template on the PC desktop 4. Plug in the electrode connectors, SS2L, into channel 1 of the MP35 data acquisition system. Double click on the BE Lab (Biopac) template: The template shows three graphs on the top half of the screen, the first is the EMG readings in mV, the second is the force exerted on the dynamometer, measured in Kg, and the third represents a continuous integral of the first EMG graph, measured in Volts. 5. Plug in the hand dynamometer, SS25L, to channel 2. 6. Make sure the switch on the back of the MP35 box is turned on. 7. Calibrate Channel 2 for the hand dynamometer. In the calibration procedure, you select the sensors and set hardware internal parameters such as gain, offset and scaling which optimize the signal. Follow the on-screen directions to calibrate the hand dynamometer. CONTINUED ON NEXT PAGE 8 8. Click on Start. The start button is located in the lower right hand corner of the screen. 9. Clench, release and wait and Keep the wrist and arm position the same for all repeat at 4-5 different levels to clenches. Grip the dynamometer close to the see your maximum possible force. crossbar but do not actually touch the crossbar. Figure 5 10. Click on Stop. 11. If data looks correct, repeat this 2 more times, for a total of THREE runs for this subject. Then go to step 13. Your data should resemble the following figure. Figure 6 12. If data looks incorrect, go to step 8. To reset graphs, go to EDIT -> Select All and EDIT -> Clear CONTINUED ON NEXT PAGE Note: If you can’t see the maximum values of your data, single-click on the right-hand axis. Increase the scale value until you can see all of your data. Alternatively, you can select the vertical autoscale button under the menus. Doing this will not interrupt your recording. Go back to step 8 and repeat if your data is not correct. 9 EXPERIMENT 2 13. Click on Start to append to Experiment 1 data. 14. Clench the hand dynamometer Note the maximum clench force so you can with your maximum force. Note determine when the force has decreased by 50%. this force and try to maintain it. Try to maintain the maximum force even though the muscle will fatigue and the force will decrease. The maximum force and fatigue time will vary greatly among subjects. 15. When the maximum clench force has decreased more than 50%, click on Stop. 16. Review the data on the screen. Your data should look like the following figure. Figure 7 You may need to use the horizontal scroll bar to view the data. You can also select the time range displayed on the x-axis. 17. If data is correct, save the data on the C drive as a BSL Profile (*ACQ). Each subject will need a unique file name. Then repeat Experiments 1 and 2 for all members of your group. END OF FIRST RECORDING 10 EXPERIMENT 3 1. In ONE subject, perform Experiment 2 (clench and hold) on the dominant arm THREE times, allowing a 1 minute rest period between runs. 2. Attach leads to the nondominant arm of that subject, and measure the arm circumference, as described in Step 3 of SET UP. 3. Click Start to append to the Experiment 3 file, and perform Experiment 2 (clench and hold) on the non-dominant arm THREE times, allowing a 1 minute rest period between runs 4. Review the data on the screen. 5. If data is correct, use “Save as…” to save the data on the C drive as a BSL Profile (*ACQ) file using REPEATED in the filename. You may need to use the horizontal scroll bar to view the data. You can also select the time range displayed on the x-axis. DO NOT USE “Save..” as it will overwrite previous file. END OF LAST RECORDING 11 V. DATA ANALYSIS The Data Analysis should be done in a M204 or any computer lab in school of engineering computer labs. FAST TRACK RECORDING Detailed Explanation for Analyzing Lesson Data 1. Open BIOPAC Pro and then open your saved data file. For the first part of the analysis, choose the data for the first subject. 2. Set up your display for optimal viewing of the first data segment. The following tools help you adjust the data window. Autoscale horizontal Autoscale waveform Zoom Tool Horizontal Scroll Bar Vertical Scroll Bar Zoom Preview 3. Using the I-Beam cursor, select an area of the plateau of the first clench and determine the force and integrated EMG. Figure 8 4. Repeat step 3 on the plateau of each successive clench and extract force and integrate EMG data for all 3 runs from each subject. 5. Go to Experiment 2 of the data. Using the I-Beam cursor, select a point in the region of maximum clench force and note the value. The point selected should represent the maximal clench force at the start of Experiment 2. Figure 9 CONTINUED ON NEXT PAGE 12 6. Calculate 50% of the maximum clench force. 7. Find the segment when the force is 50% of the maximum clench force with the I-Beam cursor. Make an eyeball approximation of the point that is 50% of the maximal clench force. Then use the Ibeam cursor in this region and note the value displayed in the measurement box. Move the cursor until you are within 5% of the desired value and leave the cursor there. 8. Select the area from the point of 50% clench force back to the maximum clench force using the I-beam cursor. Note this will yield the time to fatigue and extract that value. The cursor should be flashing on the point of 50% of the maximal clench force. Hold down the mouse button and drag to the left of this point until you reach the point of maximal clench force. Then release the mouse button. Figure 10 9. Save the data file. 10. Repeat analysis for the next subject data file. 11. Repeat analysis steps 5-9 for all runs in Experiment 3. Be sure to clearly document which values came from the dominant arm and which from the nondominant arm. 12. Exit the program. END OF ANALYSIS 13 VI. Laboratory Report Check guidelines at http://www.seas.upenn.edu/~mtsi/mtsireport.pdf Your individual laboratory report should be 3-4 pages of text plus tables and figures. You may work together with your group members on the Results table and plot, but all of the other work should be done on an individual basis. 1. Items to include in procedure A brief description of the methods and procedures used in the experiment is given here. 2. Items to include in the Results section The results are presented quantitatively in tables and/or graphs. When plotting experimental data points, plot individual data points and do not connect them with a line unless you have a specific reason for doing so. Be sure to paste your figures from Excel properly so that the entire spreadsheet is not included with the figure. You should comment on significant aspects of the results and trends in the data but not go into a detailed discussion of the analysis in this section. a. Description of subjects (male, female, dominant arm circumference, level of regular physical activity, etc.) and the maximum clench force and fatigue time (the time for the maximum clench force to decay 50%) from Experiment 2 in a table. b. Plot the plateau of the clench force and integrated EMG values from Experiment 1 for each subject on one graph. Show all 3 runs for each subject. Clearly indicate which values are from which run from a single subject, and distinguish one subject’s data from another. 3. Questions to address in the Discussion section Here you analyze your results and what they mean. The data discussed here should have already appeared elsewhere in the Results section. a. Explain the source of the signals detected from the EMG electrodes. b. Is there a difference in the maximum clench force generated by males and females in your group? (if you have female members in your group) What might explain the difference? What other factors may influence the maximum clench strength? c. As you fatigue, the force exerted by your muscles decreases. What physiological processes explain the decline in strength? How did fatigue time 14 vary across subjects and why? Between the dominant arm and non-dominant arm? Between the first and third run in Experiment 3? d. On your graph of all subjects, relate the integrated EMG signal to the corresponding plateau of the clench force measured in Experiment 1. What is the meaning of the slope? Determine the slope between EMG plateau value (y) and clench force (x). How does the slope vary with subject? Why? e. Are there experimental factors that might influence the integrated EMG value or clench force? f. What do your results indicate about the usefulness of EMG in the monitoring of athletic training? 4. Conclusion Section The Conclusion is a short summary of what you have already said. There is no new information here, just a brief presentation of the most significant points already mentioned. It should summarize the work concisely and completely. 15