Scientific abstract
advertisement
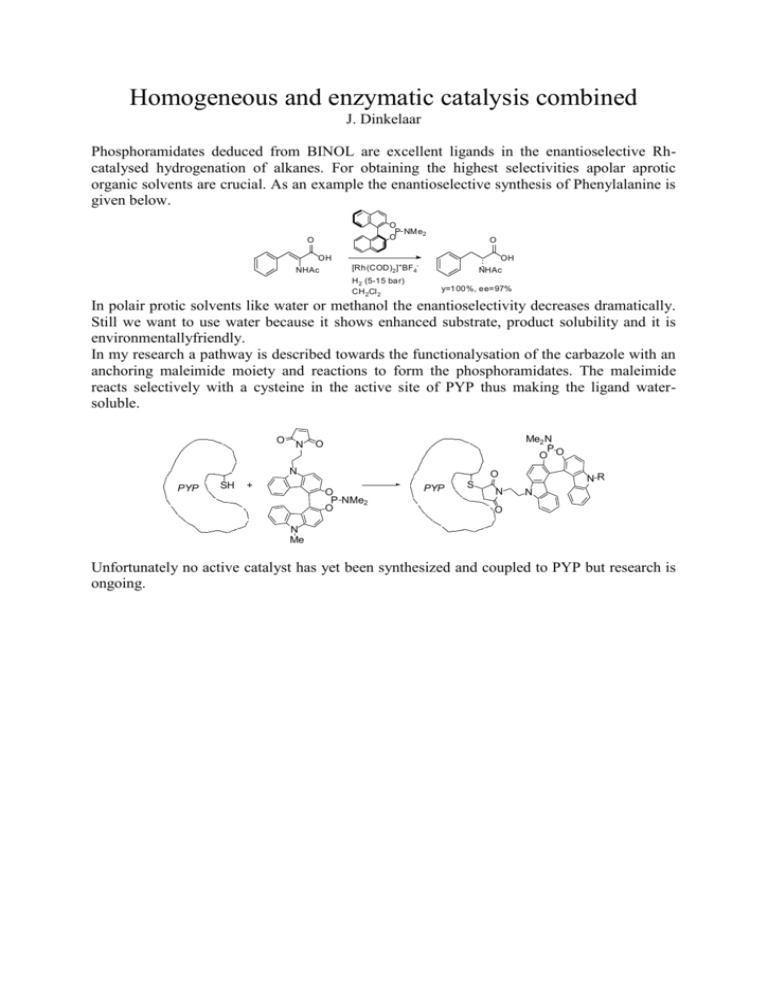
Homogeneous and enzymatic catalysis combined J. Dinkelaar Phosphoramidates deduced from BINOL are excellent ligands in the enantioselective Rhcatalysed hydrogenation of alkanes. For obtaining the highest selectivities apolar aprotic organic solvents are crucial. As an example the enantioselective synthesis of Phenylalanine is given below. O P NMe2 O O OH NHAc O OH NHAc [Rh(COD)2]+BF4H 2 (5-15 bar) CH 2Cl 2 y=100%, ee=97% In polair protic solvents like water or methanol the enantioselectivity decreases dramatically. Still we want to use water because it shows enhanced substrate, product solubility and it is environmentallyfriendly. In my research a pathway is described towards the functionalysation of the carbazole with an anchoring maleimide moiety and reactions to form the phosphoramidates. The maleimide reacts selectively with a cysteine in the active site of PYP thus making the ligand watersoluble. O N Me2 N P O O O N PYP SH + O O P NMe2 O PYP S N NR N O N Me Unfortunately no active catalyst has yet been synthesized and coupled to PYP but research is ongoing.











