Lab 1 standard solutions
advertisement

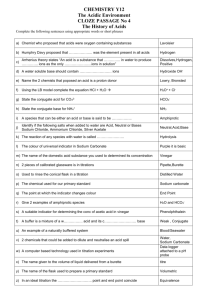
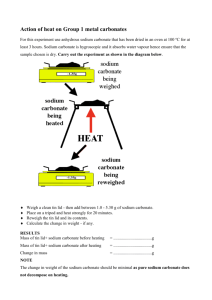
CVEG 5234 Water and Wastewater Analysis Lab #1 Standard Solutions (chapter 15) Spring 2007 Tuesday, January 16 and 23, 2007 Objective: To learn about standard solutions and to experience instrument calibration and solution titration. A standard solution is one whose strength or reacting value per unit of volume (concentration, molarity, or normality) is known. Primary standards are usually salts or acids of high purity that can be dried at some convenient temperature without decomposing and that can be weighed with a high degree of accuracy. Secondary standard solutions are solutions that have been standardized against a primary standard. Preparation of N/1 Sulfuric Acid: For the lab, we will standardize sulfuric acid (secondary) against sodium carbonate (primary). We'll make up some sulfuric acid a little stronger than 1 N and some sodium carbonate to standardize it against. Then we'll titrate the sulfuric acid into the sodium carbonate solution. By monitoring pH, we can tell when we're close to the point where the stuff neutralizes eachother. We'll put methyl orange in it, and when it is neutralized, it will change color. Then we can calculate the normality and dilute to 1 N. 1. Preparation of N/1 acid: get a volume or mass of concentrated sulfuric acid, that when diluted to 1L will give a concentration 5-10% stronger than 1 N. Then dilute to 1 L. Note: "do as you oughta, add acid to water" - have flask ~ 1/2 full with water first. 2. Decide: What volume of sodium carbonate solution you want to use, and how much titrated acid would be convenient for the test. 3. Based on your decision above, calculate the normality of sodium carbonate solution that will allow you to use your desired volume of solution and titrant, i.e., the volume of sodium carbonate solution will have the same equivalents as the volume of titrated acid. Try to find a beaker that can be ~sealed with a watch glass. 4. Make the sodium carbonate solution. Accuracy on the weight and volume is very important because this is your primary standard. 5. Titrate the acid into the sodium carbonate to a pH of ~5, then lift out the pH probe and rinse the probe into the beaker. 6. Boil gently for 3 to 5 minutes under a watch glass. Don't allow any moisture to escape. (I think this drives off the CO2 or something). Cool to ~ room temperature and rinse the cover glass into the beaker. 7. Finish titrating to the methyl orange endpoint. 8. Calculate the normality of the acid. If you figured out step 3, you can figure this out. 9. Dilute the acid to 1 Normal. (Note: The volume you have is the volume you made minus what you took out for titration. Add a calculated volume of DI water to get it to 1 N.) 10. Titrate again? (time permitting)