Aldol Condensation
advertisement

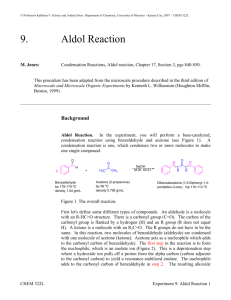
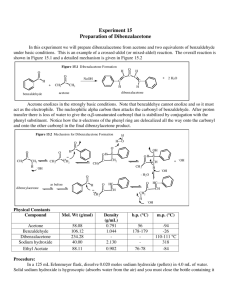
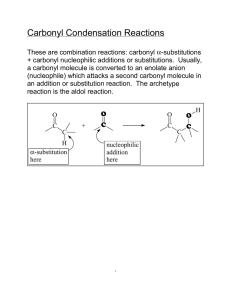
Aldol Condensation The acidity of organic compounds is often determined by neighboring groups because they can help stabilizing the resulting anion (i.e., halogen, nitro, etc.) because of their electronegative character Functional group pKa Alkane ~50 Ester ~25 Aldehyde/ketone ~18-20 Alcohol ~15-19 For instance, the presence of a carbonyl group greatly increases the acidity of neighboring hydrogen atoms (a-protons) because of the resonance stabilization in the resulting enolate ion (the numbers in parentheses below are from acetone, AM1) 127.8 pm (123.5 pm) 137.4 pm (149.5 pm) Many of the carbonyl compounds can be deprotonated with moderately strong bases i.e., hydroxide, alcoholates, etc. Ketones and aldehydes can be reacted with each other in Aldol or Claisen-Schmidt condensation H + O Ph H O H O O OH O T H H + O [OH-] Aldol H Self condensation of acetaldehyde to O alsoOH form[OH crotonaldehyde called "aldol" T ] H Ph Formation of cinnamaldehyde O H Ph In Chem 14CL, acetone is reacted with two equivalents of benzaldehyde using sodium hydroxide as catalyst O CHO O 2 NaOH(aq.)/EtOH + 2 H2O + The first step is the formation of the first enolate ion O C O C + –OH C O C C C + H2O H en olate ion Note that water is one of the products in the enolate formation water has to be excluded from the reaction mixture as much as possible (dry glassware, absolute ethanol) in order to optimize the amount of enolate The enolate ion acts as the nucleophile, which attacks the carbonyl group of the benzaldehyde to form the new C-C bond The trans, trans-isomer is primarily formed and isolated in the reaction Acetone is reacted with two equivalents of benzaldehyde in a small Erlenmeyer flask An aqueous ethanolic solution of sodium hydroxide is added slowly (drop wise) while stirring The mixture is stirred for 30 minutes The crude product precipitates as a yellow solid from the solution during the reaction The solid is isolated by vacuum filtration The filter flask has to be clamped The neoprene adapter (rubber adapter) has to be placed between the porcelain funnel and the filter flask to have a better seal A filter paper is placed on the plate of the funnel so that all holes of the porous filter plate are covered The stir bar is removed from the reaction mixture and rinsed with water After the suction is turned on, the filter paper is wetted with water to ensure that the filter paper adheres to the plate The suspension is carefully poured into the funnel The solids that are stuck to the walls is scraped off and suspended in a small amount of water to transfer it to the funnel as well The filter cake is rinsed with small portions of water until the filtrate is neutral (test with pH-paper) The crude contains benzaldehyde, benzalacetone (mono-addition product), dibenzalacetone, mesityl oxide (self-condensation product of acetone), etc. The crude product is purified by recrystallization The literature suggest ethyl acetate or ethanol as solvent for the recrystallization In Chem 14CL, 95 % ethanol is used for this purpose because dibenzalacetone is the least polar compound in the mixture and therefore displays the lowest solubility in 95 % ethanol Slow crystallization yields nicer crystals and a purer product Procedure The crude is dissolved in a MINIMUM amount of the boiling solvent Place the solid and a stir bar in a small Erlenmeyer flask Add a small amount of solvent to the solid While stirring, heat the mixture up to a gentle boil to dissolve the solid If the solid does not dissolve completely, add a little more solvent and boil again Any solids that do not dissolve in the hot solution are removed by filtration The hot, saturated solution is allowed to cool down to room temperature slowly The solution is then placed in an ice-bath for 15 minutes to reduce the solubility of the compound in the solution The product is isolated by vacuum filtration and rinsed with a small amount of water Air is sucked through the solid for 15 minutes to pre-dry the solid Transfer the product to a vial and leave it open during the week to dry the product The melting point (m.p.) of a pure substance is defined as the temperature at which both the solid and liquid phases of that substance has the SAME vapor pressure i.e., both phases are in equilibrium Melting points are used: to identify compounds (by comparison with literature values) to establish purity the purer the sample the higher the melting point the narrower the melting point range impure samples display a melting point depression and a broad melting point range while a pure substance usually possesses a very sharp melting point (T=0.5-1 oC) Exception: Eutetic mixtures possess a sharp melting point as well Acetone and benzaldehyde are colorless liquids while the product of the reaction is bright yellow. Why? Acetone only possesses an isolated carbonyl group In benzaldehyde, the carbonyl group is conjugated to a benzene ring In the product, the carbonyl group is connected to two alkene functions that are attached to benzene rings The conjugation decreases the HOMO-LUMO gap resulting in a bathochromic shift for the transition (HOMO-LUMO gap calculations with DFT, EDF2, 6-31**) Compound l (nm) e(M-1*cm-1) CH3COCH3 188 900 6.01 PhCHO 244 14100 4.90 trans-PhCH=CHCOCH3 286 32360 4.27 trans, trans-PhCH=CHCOCH=CHPh 330 34300 3.84 cis, trans-PhCH=CHCOCH=CHPh 295 20000 cis, cis-PhCH=CHCOCH=CHPh 287 11000 HOMO-LUMO GAP (eV)