organic chemistry experiment ii functional groups
advertisement
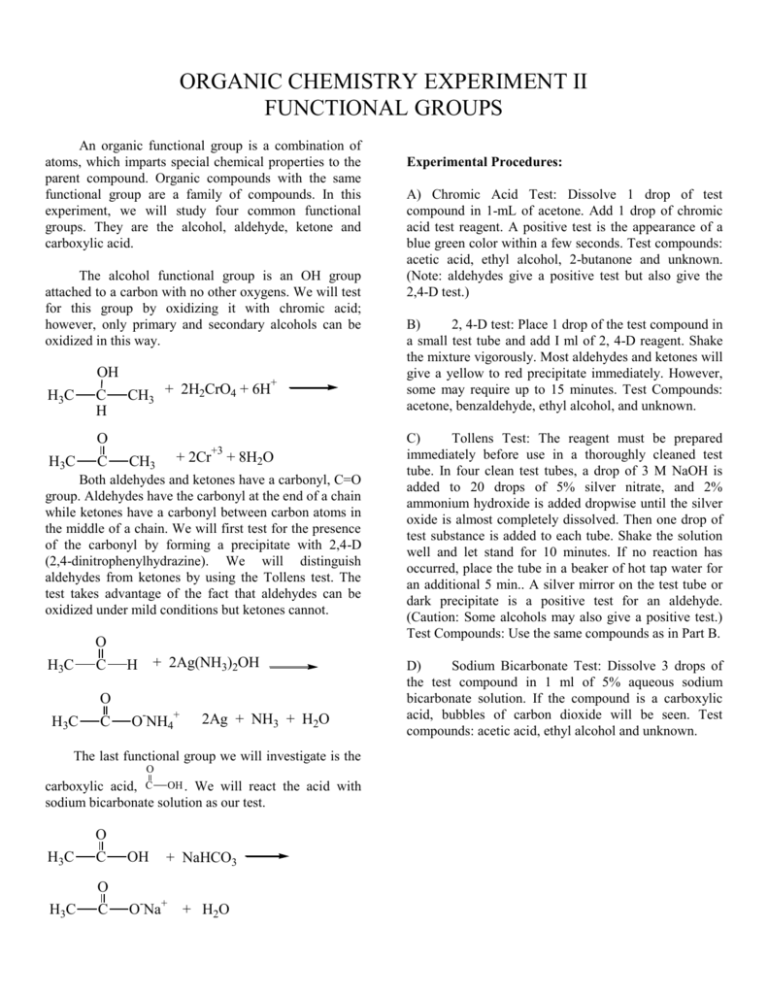
ORGANIC CHEMISTRY EXPERIMENT II FUNCTIONAL GROUPS An organic functional group is a combination of atoms, which imparts special chemical properties to the parent compound. Organic compounds with the same functional group are a family of compounds. In this experiment, we will study four common functional groups. They are the alcohol, aldehyde, ketone and carboxylic acid. The alcohol functional group is an OH group attached to a carbon with no other oxygens. We will test for this group by oxidizing it with chromic acid; however, only primary and secondary alcohols can be oxidized in this way. OH H3C C H + CH3 + 2H2CrO4 + 6H O H3C C +3 + 2Cr CH3 + 8H2O Both aldehydes and ketones have a carbonyl, C=O group. Aldehydes have the carbonyl at the end of a chain while ketones have a carbonyl between carbon atoms in the middle of a chain. We will first test for the presence of the carbonyl by forming a precipitate with 2,4-D (2,4-dinitrophenylhydrazine). We will distinguish aldehydes from ketones by using the Tollens test. The test takes advantage of the fact that aldehydes can be oxidized under mild conditions but ketones cannot. O H3C C + 2Ag(NH3)2OH H O H3C C - + O NH4 2Ag + NH3 + H2O The last functional group we will investigate is the O carboxylic acid, C OH . We will react the acid with sodium bicarbonate solution as our test. O H3C C OH + NaHCO3 O H3C C O-Na+ + H2O Experimental Procedures: A) Chromic Acid Test: Dissolve 1 drop of test compound in 1-mL of acetone. Add 1 drop of chromic acid test reagent. A positive test is the appearance of a blue green color within a few seconds. Test compounds: acetic acid, ethyl alcohol, 2-butanone and unknown. (Note: aldehydes give a positive test but also give the 2,4-D test.) B) 2, 4-D test: Place 1 drop of the test compound in a small test tube and add I ml of 2, 4-D reagent. Shake the mixture vigorously. Most aldehydes and ketones will give a yellow to red precipitate immediately. However, some may require up to 15 minutes. Test Compounds: acetone, benzaldehyde, ethyl alcohol, and unknown. C) Tollens Test: The reagent must be prepared immediately before use in a thoroughly cleaned test tube. In four clean test tubes, a drop of 3 M NaOH is added to 20 drops of 5% silver nitrate, and 2% ammonium hydroxide is added dropwise until the silver oxide is almost completely dissolved. Then one drop of test substance is added to each tube. Shake the solution well and let stand for 10 minutes. If no reaction has occurred, place the tube in a beaker of hot tap water for an additional 5 min.. A silver mirror on the test tube or dark precipitate is a positive test for an aldehyde. (Caution: Some alcohols may also give a positive test.) Test Compounds: Use the same compounds as in Part B. D) Sodium Bicarbonate Test: Dissolve 3 drops of the test compound in 1 ml of 5% aqueous sodium bicarbonate solution. If the compound is a carboxylic acid, bubbles of carbon dioxide will be seen. Test compounds: acetic acid, ethyl alcohol and unknown. Aldehyde Boiling Points propanal 2-methylpropanal butanal 3-methylbutanal 2-butenal 2-ethylbutanal hexanal heptanal benzaldehyde Ketone Boiling Points 48° 64° 75° 92° 104° 117° 130° 153° 179° Alcohol Boiling Points methanol 2-propanol 2-butanol 1-butanol 3-methyl -1-butanol 1-hexanol 1-heptanol acetone 2-butanone 2-pentanone 4-methyl-2-pentanone cyclopentanone 2-heptanone 2-octanone 56° 80° 101° 117° 131° 151° 173° Carboxylic Acid Boiling Points 65° 82° 99° 118° 130° 157° 176° acetic acid propanoic acid 2-methylpropanoic acid 2-methylpropenoic acid 3-methylbutanoic acid 118° 141° 154° 163° 176° Name__________________________________ ORGANIC FUNCTIONAL GROUPS REPORT SHEET 1. Chromic Acid Test a. Test compounds giving a positive chromic acid test are: Give the equation for the reactions observed. (Use structural formulas for organic compounds). 2. 2, 4-D Test: Test compounds giving a positive 2, 4-D test: 3. Tollens Test: a. Test compounds giving a positive Tollens Test are: b. Give the equation for the reactions observed. 4. Sodium Bicarbonate Test: a. Test compounds giving a positive NaHCO3 are: c. Give the equations for the reactions observed. 5 Draw the structure of the following compounds: 1 –butanol 6. 2-pentanone butanal propanoic acid Unknown Number: ______________ Name of unknown: ___________________________ Boiling point: ____________ Structure of Unknown: Test Results: (List + or -) Chromic Acid _____ 2, 4-D Test _____ Tollens Test _____ NaHCO3 Test _____ Name_____________________________________ PRELABORATORY ASSIGNMENT - ORGANIC FUNCTIONAL GROUPS 1. How does an aldehyde differ from a ketone? 2. Both an alcohol and aldehyde give a positive chromic acid test. How can they be distinguished? 3. An unknown is added to aqueous NaHCO3 solution. No gas is formed. What does this mean? 4. How does the alcohol functional group differ from the carboxylic acid functional group? 5. An unknown gives a precipitate when 2, 4-D is added but does not react with Tollens reagent. The boiling point is 116±2°. The unknown is: