Cardiac electrophysiology
advertisement

BUKOVINІАN STATE MEDICAL UNIVERSITY
“Approved”
on the Methodological Meeting
of the Department of Internal medicine,
Physical rehabilitation and Sport medicine
Bukovinian State Medical University
“____” ____________________ 20___.
Minutes No_______
Deputy Chief of the Department,
Doctor of Medical Science
V.K. Tashchuk
METHODOLOGICAL INSTRUCTIONS
to the practical class on the topic:
“ARRHYTHMIAS, BLOCKADES (PAROXISMAL RHYTHM
DISORDERS) – THE LESSON IS HELD AT THE BASIS OF
CARDIOLOGICAL CLINIC.”
Chernivtsi– 2010
Topic of the Class: The heart’s primary function is to pump blood to all parts of the body,
bringing nutrients and oxygen to the tissues and removing waste products. When the body is at
rest, it needs a certain amount of blood to achieve this function. During exercise or times when
greater demands are placed on the body, more blood is required. To meet these variable
demands, the heartbeat increases or decreases, and blood vessels dilate to deliver more blood
or constrict during times when less blood is required.
2. Duration of the class: 4 .
3.Objectives:
To know:
1.
2.
3.
4.
5.
6.
7.
8.
Вe able to:
1.
2.
3.
4.
5.
6.
7.
8.
Etiology and pathogenesis of arrhythmias.
Diagnostics of arrhythmias.
Classification of arrhythmias.
Treatment of arrhythmias.
Etiology and pathogenesis of blockades.
Diagnostics of blockades.
Classification of blockades.
Treatment of blockades.
Explain etiology and pathogenesis of myocarditis.
Diagnose of arrhythmias.
Classificate of arrhythmias.
Treatment of arrhythmias.
Explain etiology and pathogenesis of blockades.
Diagnose of blockades.
Classificate of blockades.
Treatment of blockades.
4. Advice to the student.
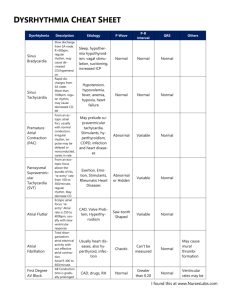
Cardiac arrhythmia is any of a group of conditions in which the electrical activity of the heart is
irregular or is faster or slower than normal.
Some arrhythmias are life-threatening medical emergencies that can cause cardiac arrest and sudden
death. Others cause aggravating symptoms, such as an awareness of a different heart beat, or palpitation,
which can be annoying. Some are quite minor and can be regarded as normal. Sinus arrhythmia is the mild
acceleration followed by slowing of the normal rhythm that occurs with breathing. In adults the normal
resting heart rate ranges from 60 beats per minute to 100 beats per minute. The normal heart beat is
controlled by a small area in the upper chamber of the heart called the sinoatrial node or sinus node. The
sinus node contains specialized cells that have spontaneous electrical activity that starts each normal heart
beat.
Faster and slower arrythmias
In an adult, a heart rate faster than 100 beats/minute is considered tachycardia. This number varies
with age, as the heartbeat of a younger person is naturally faster than that of an older person's. During
exercise the sinus node increases its rate of electrical activity to accelerate the heart rate. Such normal fast
rate that develops is called sinus tachycardia. In contrast, arrhythmias that are due to fast, abnormal electrical
activity can cause tachycardias that are dangerous. If the ventricles of the heart experience one of these
tachycardias for a long period of time, there can be deleterious effects. Individuals may sense a tachycardia
as a pounding sensation of the heart, known as palpitations. If a tachycardia lowers blood pressure it may
cause lightheadedness or dizziness, or even fainting (syncope). If the tachycardia is too fast, the pump
function of the heart is impeded, and rarely may lead to sudden death.
2
Most tachycardias are not dangerous. Anything that increases adrenaline or its effects on the heart
will increase the heart rate and potentially cause palpitations or tachycardias. Causes include stress, ingested
or injected substances (ie: caffeine, amphetamines, alcohol—see Holiday heart syndrome), and an overactive
thyroid gland (hyperthyroidism). Individuals who have a tachycardia are often advised to limit or remove
exposure to any causative agent. However, these causative agents are not the only contributors to
tachycardias and their prevalence has not been evaluated statistically.
A slow rhythm, known as bradycardia (less than 60 beats/min), is usually not life threatening, but
may cause symptoms. It may be caused by reversible causes (low oxygen, electrolyte abnormalities), or be
more permanent (heart block). When it causes symptoms implantation of a permanent pacemaker may be
needed. Either dysrhythmia requires medical attention to evaluate the risks associated with the arrhythmia.
Fibrillation
A serious variety of arrhythmia is known as fibrillation. The muscle cells of the heart normally
function together, creating a single contraction when stimulated. Fibrillation occurs when the heart muscle
begins a quivering motion due to a disunity in contractile cell function. Fibrillation can affect the atrium
(atrial fibrillation) or the ventricle (ventricular fibrillation); ventricular fibrillation is imminently lifethreatening.
Atrial fibrillation is the quivering, chaotic motion in the upper chambers of the heart, known as the
atria. Atrial fibrillation is often due to serious underlying medical conditions, and should be evaluated by a
physician. It is not typically a medical emergency.
Ventricular fibrillation occurs in the ventricles (lower chambers) of the heart; it is always a medical
emergency. If left untreated, ventricular fibrillation (VF, or V-fib) can lead to death within minutes. When a
heart goes into V-fib, effective pumping of the blood stops. V-fib is considered a form of cardiac arrest, and
an individual suffering from it will not survive unless cardiopulmonary resuscitation (CPR) and defibrillation
are provided immediately.
CPR can prolong the survival of the brain in the lack of a normal pulse, but defibrillation is the
intervention which is most likely to restore a more healthy heart rhythm. It does this by applying an electric
shock to the heart, after which sometimes the heart will revert to a rhythm that can once again pump blood.
Almost every person goes into ventricular fibrillation in the last few minutes of life as the heart
muscle reacts to diminished oxygen or general blood flow, trauma, irritants, or depression of electrical
impulses themselves from the brain.
Origin of impulse
When an electrical impulse begins in any part of the heart, it will spread throughout the myocardium
and cause a contraction; see Electrical conduction system of the heart. Abnormal impulses can begin by one
of two mechanisms: automaticity or reentry.
Automaticity
Automaticity refers to a cardiac muscle cell firing off an impulse on its own. Every cardiac cell has
this potential: if it does not receive any impulses from elsewhere, its internal "pacemaker" will fire off an
impulse after a certain amount of time. A single specialized location in the atrium, the sinoatrial node, has a
higher automaticity (a faster pacemaker) than the rest of the heart, and therefore is usually the one to start the
heartbeat.
Any part of the heart that initiates an impulse without waiting for the sinoatrial node is called an
ectopic focus, and is by definition a pathological phenomenon. This may cause a single premature beat now
and then, or, if the ectopic focus fires more often than the sinoatrial node, it can produce a sustained
abnormal rhythm. Rhythms produced by an ectopic focus in the atria, or by the atrioventricular node, are the
least dangerous dysrhythmias; but they can still produce a decrease in the heart's pumping efficiency,
because the signal reaches the various parts of the heart muscle with slightly different timing than usual and
causes a poorly coordinated contraction.
Conditions that increase automaticity include sympathetic nervous system stimulation and hypoxia.
The resulting heart rhythm depends on where the first signal begins: if it is the sinoatrial node, the rhythm
remains normal but rapid; if it is an ectopic focus, many types of dysrhythmia can result.
Re-entry
Re-entry dysrhythmias occur when an electrical impulse travels in a circle within the heart, rather
than moving outward and then stopping. Every cardiac cell is able to transmit impulses in every direction,
but will only do so once within a short period of time. Normally the impulse spreads through the heart
quickly enough that each cell will only respond once, but if conduction is abnormally slow in some areas,
part of the impulse will arrive late and will be treated as a new impulse, which can then spread backward.
3
Depending on the timing, this can produce a sustained abnormal rhythm, such as atrial flutter, a self-limiting
burst of supraventricular tachycardia, or the dangerous ventricular tachycardia.
Diagnosis
Cardiac dysrhythmias are often first detected by simple but nonspecific means: auscultation of the
heartbeat with a stethoscope, or feeling for peripheral pulses. These cannot usually diagnose specific
dysrhythmias, but can give a general indication of the heart rate and whether it is regular or irregular. Not all
the electrical impulses of the heart produce audible or palpable beats; in many cardiac arrhythmias, the
premature or abnormal beats do not produce an effective pumping action and are experienced as "skipped"
beats.
The simplest specific diagnostic test for assessment of heart rhythm is the electrocardiogram. A
Holter monitor is an EKG recorded over a 24-hour period, to detect dysrhythmias that may happen briefly
and unpredictably throughout the day.
Cardiac action potential
The cardiac action potential is a specialized action potential in the heart, with unique properties
necessary for function of the electrical conduction system of the heart.
The cardiac action potential has five phases.
The cardiac action potential differs significantly in different portions of the heart. This differentiation
of the action potentials allows the different electrical characteristics of the different portions of the heart. For
instance, the specialized conduction tissue of the heart has the special property of depolarizing without any
external influence. This is known as automaticity.
The electrical activity of the specialized conduction tissues are not apparent on the surface
electrocardiogram (ECG). This is due to the relatively small mass of these tissues compared to the
myocardium.
Cardiac muscle has some similarities to neurons and skeletal muscle, as well as important unique
properties. Like a neuron, a given myocardial cell has a negative membrane potential when at rest.
Stimulation above a threshold value induces the opening of voltage-gated ion channels and a flood of cations
into the cell. When the threshold is met, an action potential initiates. This causes the positively charged ions
to enter the cell [depolarization]. Like skeletal muscle, depolarization causes the opening of voltage-gated
calcium channels and entry of Ca2+ from the t-tubules. This influx of calcium causes calcium-induced
calcium release from the sarcoplasmic reticulum, and the increase in myoplasmic free Ca2+ concentration
causes muscle contraction. After a delay (the absolute refractory period), Potassium channels reopen and the
resulting flow of K+ out of the cell causes repolarization to the resting state.
Note that there are important physiological differences between nodal cells and ventricular cells; the
specific differences in ion channels and mechanisms of polarization give rise to unique properties of SA
node cells, most importantly the spontaneous depolarizations (automaticity) necessary for the SA node's
pacemaker activity.
Calcium channels
Two voltage-dependent calcium channels play critical roles in the physiology of cardiac muscle: Ltype calcium channel ('L' for Long-lasting) and T-type calcium channels ('T' for Transient) voltage-gated
calcium channels.
These channels respond differently to voltage changes across the membrane: L-type channels
respond to higher membrane potentials, open more slowly, and remain open longer than T-type channels.
Because of these properties, L-type channels are important in sustaining an action potential, while Ttype channels are important in initiating them.
4
Because of their rapid kinetics, T-type channels respond better to rhythmic stimulation and are also
found in some neuron cell bodies, where they play an important role in rhythmic processes such as heartbeat,
breathing, and spinal cord pattern generators used in walking.
L-type channels are selectively blocked by dihydropyridines.
Resting membrane potential
The resting membrane potential is caused by the difference in ionic concentrations and conductances
across the membrane of the cell during phase 4 of the action potential. The normal resting membrane
potential in the ventricular myocardium is about -85 to -95 mV. This potential is determined by the selective
permeability of the cell membrane to various ions. The membrane is most permeable to K+ and relatively
impermeable to other ions. The resting membrane potential is therefore dominated by the K+ equilibrium
potential according to the K+ gradient across the cell membrane. The membrane potential can be calculated
using the Goldman equation|Goldman-Hodgkin-Katz voltage equation. The maintenance of this electrical
gradient is due to various ion pumps and exchange mechanisms, including the Na+-K+ ion exchange pump,
the Na+-Ca2+ exchanger current and the IK1 inwardly rectifying K+ current.
Intracellularly (within the cell), K+ is the principal cation, and phosphate and the conjugate bases of
organic acids are the dominant anions. Extracellularly (outside the cell), Na+ and Cl- predominate.
Phases of the cardiac action potential
The standard model used to understand the cardiac action potential is the action potential of the
ventricular myocyte. The action potential has 5 phases (numbered 0-4). Phase 4 is the resting membrane
potential, and describes the membrane potential when the cell is not being stimulated.
Once the cell is electrically stimulated (typically by an electric current from an adjacent cell), it
begins a sequence of actions involving the influx and efflux of multiple cations and anions that together
produce the action potential of the cell, propagating the electrical stimulation to the cells that lie adjacent to
it. In this fashion, an electrical stimulation is conducted from one cell to all the cells that are adjacent to it, to
all the cells of the heart.
Phase 4
Phase 4 is the resting membrane potential. This is the period that the cell remains in until it is
stimulated by an external electrical stimulus (typically an adjacent cell). This phase of the action potential is
associated with diastole of the chamber of the heart.
Certain cells of the heart have the ability to undergo spontaneous depolarization, in which an action
potential is generated without any influence from nearby cells. This is also known as automaticity. The cells
that can undergo spontaneous depolarization the fastest are the primary pacemaker cells of the heart, and set
the heart rate. Usually, these are cells in the SA node of the heart. Electrical activity that originates from the
SA node is propagated to the rest of the heart. The fastest conduction of electrical activity is via the electrical
conduction system of the heart.
In cases of heart block, in which the activity of the primary pacemaker does not propagate to the rest
of the heart, a latent pacemaker (also known as an escape pacemaker) will undergo spontaneous
depolarization and create an action potential.
The mechanism of automaticity involves the so-called pacemaker channels of the HCN family,
Hyperpolarization-gated, Cyclic Nucleotide-gated channels. These poorly selective cation channels conduct
more current as the membrane potential becomes more negative, or hyperpolarized. They conduct both
potassium and sodium. The activity of these channels in the SA node cells causes the membrane potential to
slowly become more positive (depolarized) until, eventually, calcium channels are activated and an action
potential is initiated.
Phase 0
Phase 0 is the rapid depolarization phase. The slope of phase 0 represents the maximum rate of
depolarization of the cell and is known as Vmax. This phase is due to the opening of the fast Na+ channels
causing a rapid increase in the membrane conductance to Na+ (GNa) and thus a rapid influx of Na+ ions (INa)
into the cell; a Na+ current.
The ability of the cell to open the fast Na+ channels during phase 0 is related to the membrane
potential at the moment of excitation. If the membrane potential is at its baseline (about -85 mV), all the fast
Na+ channels are closed, and excitation will open them all, causing a large influx of Na + ions. If, however,
the membrane potential is less negative, some of the fast Na + channels will be in an inactivated state
insensitive to opening, thus causing a lesser response to excitation of the cell membrane and a lower Vmax.
For this reason, if the resting membrane potential becomes too positive, the cell may not be excitable, and
conduction through the heart may be delayed, increasing the risk for arrhythmias.
5
The fast Na+ channel
The fast sodium channel can be modeled as being controlled by a number of gates. Each gate (or
gating variable) can attain a value between 1 (fully open) and 0 (fully closed). The product of all the gates
denotes the percentage of channels available to conduct Na+. Following the model of Hodgkin and Huxley,
the sodium channel contains three gates: m, h, and j. In the resting state, the m gate is closed (zero) and the h
and j gates are open (one). Hence, the product denoting the percentage of conducting channels is also zero.
Upon electrical stimulation of the cell, the m gate opens quickly while simultaneously the h and j gates close
more slowly. For a brief period of time, all gates are open (i.e. non-zero) and Na+ can enter the cell following
its electrochemical gradient. If, as above, the resting membrane potential is too positive, the h or j gates may
be considerably less than one, such that the product of m, h and j becomes too small upon depolarization.
Phase 1
Phase 1 of the action potential occurs with the inactivation of the fast Na+ channels. The transient net
outward current causing the small downward deflection of the action potential is due to the movement of K +
and Cl- ions, carried by the Ito1 and Ito2 currents, respectively. Particularly the Ito1 contributes to the "notch" of
some ventricular cardiomyocyte action potentials.
It has been suggested that Cl- ions movement across the cell membrane during Phase I is as a result
of the change in membrane potential, from K+ efflux, and is not a contributory factor to the initial
repolarisation ("notch").
Phase 2
This "plateau" phase of the cardiac action potential is sustained by a balance between inward
movement of Ca2+ (ICa) through L-type calcium channels and outward movement of K+ through the slow
delayed rectifier potassium channels, IKs. The sodium-calcium exchanger current, INa,Ca and the
sodium/potassium pump current, INa,K also play minor roles during phase 2.
Phase 3
During phase 3 of the action potential, the L-type Ca2+ channels close, while the slow delayed
rectifier (IKs) K+ channels are still open. This ensures a net outward current, corresponding to negative
change in membrane potential, thus allowing more types of K+ channels to open. These are primarily the
rapid delayed rectifier K+ channels (IKr) and the inwardly rectifiyng K+ current, IK1. This net outward,
positive current (equal to loss of positive charge from the cell) causes the cell to repolarize. The delayed
rectifier K+ channels close when the membrane potential is restored to about -80 to -85 mV, while IK1
remains conducting throughout phase 4, contributing to set the resting membrane potential.
Cardiac electrophysiology
Cardiac electrophysiology (or electrophysiology) is the science of the mechanisms, functions, and
performance of the electrical activities of specific regions of the heart.
An electrophysiologic study is a term used to describe a number of invasive (intracardiac) and noninvasive recording of spontaneous electrical activity as well as of cardiac responses to programmed electrical
stimulation. These studies are performed to assess arrhythmias, elucidate symptoms, evaluate abnormal
electrocardiograms, assess risk of developing arrhythmias in the future, and design treatment. These
procedures increasingly include therapeutic methods (typically radiofrequency ablation) in addition to
diagnostic and prognostic procedures. Other therapeutic modalities employed in this field include
antiarrhythmic drug therapy and implantation of pacemakers and implantable cardioverter-defibrillators.
A specialist in cardiac electrophysiology is known as a cardiac electrophysiologist, or (more
commonly) simply an electrophysiologist. Cardiac electrophysiology is considered a subspecialty of
cardiology, and in most countries requires two or more years of fellowship training beyond a general
cardiology fellowship. They are trained to perform interventional cardiac EP procedures as well as surgical
device implantations.
Diagnostic testing
Ambulatory electrocardiographic monitoring - Holter recording and interpretation, loop recording and
interpretation;
Tilt table testing;
Signal-averaged electrocardiogram (SAECG) interpretation, also referred to as "late potentials" reading;
Electrophysiologic study (EPS) consists in the insertion of pacing and recording electrodes either in the
oesophagus (intra-oesophageal EPS) or, through blood vessels, directly into the heart chambers (intracardiac EPS) in order to measure electrical properties of the heart and, in the case of intra-cardiac EPS,
to electrically stimulate it in the attempt to induce arrhythmias for diagnostic purposes ("programmed
electrical stimulation").
Abnormal automaticity
6
The normal activity of the pacemaker cells of the heart is to spontaneously depolarize at a regular
rhythm, generating the normal heart rate. Abnormal automaticity involves the abnormal spontaneous
depolarization of cells of the heart. This typically causes arrhythmias (irregular rhythms) in the heart.
Mechanism of Arrhythmias
Mechanism of cardiac arrhytmias are divided into 2 main categories
1.
Disorders of Impulse formation
2.
Disorders of Impulse conduction
Disorders of Impulse formation
There are two major causes of impulse formation that could result in arrythmias
Automaticity Activity
Triggered Activity
Automaticity Activity
Automaticity is a characteristic of cardiac cells to undergo spontaneous diastolic depolarization and
initiate an electrical impulse in the absence of external electrical stimulation. Normal automaticity makes the
sinus node the primary pacemaker of the heart. The sinus nodal discharge rate dominates latent pacemakers
because it depolarizes more rapidly. This mechanism has been referred as overdrive supression. Examples of
arrhytmias with normal automaticity are sinus tachycardia or bradycardia inappropriate for a particular
clinical situation. This arrhythmia is caused by an alteration in the rate of impulse initiation by the normal
sinus node pacemaker without shifting the impulse origin to a subsidiary pacemaker at an ectopic site.
(Heart)
Abnormal automaticity occurs in cardiac cells when there are abnormal changes in the
transmembrane potentials due to disease or interventions. The spontaneous action potentiasl generated by
this mechanism may be caused by either Na+ or Ca2+ inward currents and sometimes a mixture of both.
Increase cathecolamines, electrolyte abnomalities and medications could all enhance automaticity and lead to
arrythmias. An example of abnormal automaticity would be accelerate idioventricular rhythm.
Normal or abnormal automaticity would also initiate arrythmias caused by nonautomatic mechanism.
For example, premature beats, caused by automaticity , can lead to reentry.
This arrythmias cannot be stopped or started by pacing.
Triggered Activity
This scenario refers to a pacemaker activity that is initiated secondary to afterdepolarizations from a
prior impulse or series of impulses. Afterdepolarizations is an oscillation in membrane potential that occurs
after repolarization
Two types of after depolarizations can be identified:
Early afterdepolarizations (EAD): they arise during the repolarization of the action potential.
They can occur with electrolyte abnormalities, acidosis, hypoxemia and increased cathecolamine states. EAD
might be responsible for the ventricular arrhytmias of the acquired and congenital forms of long QT
syndrome and also in the origen of arrhytmias in heart failure and hyperthrophy. Early depolarizations are
supressed by magnesium.
Delayed Afterdepolarizations (DAD): they occur after repolarization is completed.This
activity is seen in conditions that increase intracellular calcium. When these delayed afterdepolarizations
reach the threshold potential, it "triggers" an action potential. DAD seems to be responsible for arrhytmias of
digitalis toxicity and in the failing heart. Drugs that block calcium influx (betablockers, calcium channel
blockers) and drugs that decrease sodium current (lidocaine) supress DAD.
Disorders of Impulse Conduction
In normal conditions, the excitation wavefront initiated in the sinus node will activate the cardiac
tissue in an organized sequence and then will die out. However, there are cases where the original impulse
perpetuates and propagates itself because it always finds excitable myocardial tissue.
Reentry
This is probably the mechanism responsible for the majority of important arrhytmias. It needs two
main components so it can occur:
Two functionally distinct (different in velocity of conduction or refractroy period)
conducting pathways
Unidirectional block in one of the pathways
Of note, the time for conduction within the depressed but unblocked area must exceed the refractroy
period of the initially blocked pathway and proximal tissue. If this conditions are achieved, it will allow a
repetitive circulation of the impulse over a loop inducing the arrythmia.
7
Common clinical examples are atrial fibrillation, atrial flutter, AVNRT (AV nodal reentry
tachycardia) and WPW (Wolf Parkinson White)
Diseases of the conduction system and bradyarrhythmias
Bradyarrhythmias can be broadly divided into two categories: those at the atrial level that are
caused by sinus node dysfunction, and those at the subatrial level resulting in AV conduction disturbances.
1. Sinus Node Dysfunction
Sick Sinus Syndrome (SSS)
refers to an abnormal impulse formation in the sinus node, and/or abnormal impulse conduction. It is
frequently associated with AV nodal conduction disturbances, where alternating tachycardia (more
commonly atrial fibrillation (AF) or atrial flutter, although can be seen with other supraventricular
tachycardias) and bradycardia can be seen in up to 50% of patients.
SSS is defined by the electrocardiographic criteria,as clinical symptoms are often vague.
Here is the list of findings that can be seen with SSS:
unexplained persistent bradycardia without warning
sinus arrest or sinus exit block
paroxysmal atrial fibrillation (PAF) followed by sinus arrest
SA block/arrest or severe sinus bradycardia following a cardioversion of atrial fibrillation
Alternating bradycardia and atrial tachyarrhythmias
Slow ventricular response in atrial fibrillation in patients off any nodal agents reflects AV
nodal disease that often co-insides with a significant SA nodal dysfunction and an unstable sinus rhythm
upon conversion
Inadequate sinus acceleration with exercise
Etiology
SA node dysfunction may be a result of intrinsic or extrinsic factors.
Intrinsic Factors
Idiopathic degenerative disease (fibrosis) associated with aging: most common cause overall
SA Nodal Artery Involvement
o
Atherosclerosis
o
Vasculitis
o
Embolus
Infiltrative diseases
o
Amyloidosis
o
Hemochromatosis
o
Malignancies
Inflammatory conditions
o
Myocarditis
o
Pericarditis
Myotonic Dystrophy
Freidrech’s Ataxia
Collagen Vascular Diseases
o
SLE
o
Scleroderma
After cardiac surgery:
o
Corrective Cardiac Surgery for Congenital Heart Disease yabekref1 hayesref2
ASD repair
Transposition of the great arteries
o
Transplant
Congenital/genetic SA node dysfunction
Familial SSS is rare, but some familial cases have been reported:
o
SCN5a mutations
o
HCN4 mutations
Extrinsic Factors
Drugs
o
Antiarrhythmics: classes Ia, Ic, III.
o
Antihypertensives:
o
Beta-blockers
o
Calcium Channel Blockers
8
o
o
o
o
o
o
o
o
Digitalis
Lithium: SA nodal abnormalities seen in up to 50% of patients
Dilantin
Cimetidine
Electrolyte Disturbances
Endocrine abnormalities
Inferior Myocardial Infarction (Bezold-Jarish phenomenon)
Autonomic Nervous System Influence
CSP
Vasovagal response
Miscellaneous
High Intracranial Pressure
Obstructive Sleep Apnea
Supraventricular tachycardia
A supraventricular tachycardia (SVT) is a tachycardia or rapid rhythm of the heart in which the
origin of the electrical signal is either the atria or the AV node. These rhythms, by definition, are either
initiated or maintained by the atria or the AV node. This is in contrast to ventricular tachycardias, which are
rapid rhythms that originate from the ventricles of the heart, that is, below the atria or AV node.
The most frequently seen supraventricular tachycardia is atrial fibrillation
Can be irregular or regular
Symptoms
Symptoms can come on suddenly and may go away without treatment. They can last a few minutes
or as long as 1-2 days. The rapid beating of the heart during SVT can make the heart a less effective pump so
that the cardiac output is decreased and the blood pressure drops. The following symptoms are typical with a
rapid pulse of 140-250 beats per minute:
Palpitations - The sensation of the heart racing, fluttering or pounding strongly in the chest
or the carotid arteries
Dizziness, or lightheadedness (near-faint), or fainting
Shortness of breath
Anxiety
Chest pain or sensation of tightness
Weakness in legs
Types of SVTs
Supraventricular tachycardia is properly used as a general term that encompasses a number of
different arrhythmias of the heart, each with a different mechanism of impulse maintenance. These are listed
below.
Unfortunately, the term SVT is often loosely applied to just the subgroup of AV nodal re-entrant
tachycardias.
SVTs from a SINOATRIAL source:
Sinus tachycardia
Inappropriate sinus tachycardia
Sinoatrial node reentrant tachycardia (SANRT)
SVTs from an ATRIAL source:
(Unifocal) Atrial tachycardia (AT)
Multifocal atrial tachycardia (MAT)
9
Atrial fibrillation with a rapid ventricular response
Atrial flutter with a rapid ventricular response
SVTs from an ATRIOVENTRICULAR source:
AV nodal reentrant tachycardia (AVNRT)
AV reentrant tachycardia (AVRT)
Junctional ectopic tachycardia
Diagnosis
Most supraventricular tachycardias have a narrow QRS complex on EKG, but it is important to
realise that supraventricular tachycardia with aberrant conduction (SVTAC) can produce a wide-complex
tachycardia that may mimic ventricular tachycardia (VT). In the clinical setting, it is important to determine
whether a wide-complex tachycardia is an SVT or a ventricular tachycardia, since they are treated
differently. Ventricular tachycardia has to be treated appropriately, since it can quickly degenerate to
ventricular fibrillation and death. A number of different algorithms have been devised to determine whether
a wide complex tachycardia is supraventricular or ventricular in origin.
In general, a history of structural heart disease dramatically increases the likelihood that the
tachycardia is ventricular in origin.
The individual subtypes of SVT can be distinguished from each other by certain physiological and
electrical characteristics, many of which present in the patient's EKG.
Holter monitor-Imaging with start (red arrow) and end (blue arrow) of a SV-tachycardia with a pulse
frequency of about 128/min.
Sinus tachycardia is considered "appropriate" when a reasonable stimulus, such as the
catecholamine surge associated with fright, stress, or physical activity, provokes the tachycardia. It is
distinguished by a presentation identical to a normal sinus rhythm except for its fast rate (>100 beats per
minute in adults).
Sinoatrial node reentrant tachycardia (SANRT) is caused by a reentry circuit localised to the
SA node, resulting in a normal-morphology p-wave that falls before a regular, narrow QRS complex. It is
therefore impossible to distinguish on the EKG from ordinary sinus tachycardia. It may however be
distinguished by its prompt response to Vagal manouvres.
(Unifocal) Atrial tachycardia is tachycardia resultant from one ectopic foci within the atria,
distinguished by a consistent p-wave of abnormal morphology that fall before a narrow, regular QRS
complex.
Multifocal atrial tachycardia (MAT) is tachycardia resultant from at least three ectopic foci
within the atria, distinguished by p-waves of at least three different morphologies that all fall before regular,
narrow QRS complexes.
Atrial fibrillation is not, in itself, a tachycardia, but when it is associated with a rapid
ventricular response greater than 100 beats per minute, it becomes a tachycardia. A-fib is characteristically
an "irregularly irregular rhythm" both in its atrial and ventricular depolarizations. It is distinguished by
fibrillatory p-waves that, at some point in their chaos, stimulate a response from the ventricles in the form of
irregular, narrow QRS complexes.
Atrial flutter, is caused by a re-entry rhythm in the atria, with a regular rate of about 300
beats per minute. On the EKG, this appears as a line of "sawtooth" p-waves. The AV node will not usually
conduct such a fast rate, and so the P:QRS usually involves a 2:1 or 4:1 block pattern, (though rarely 3:1, and
most rarely and sometimes fatally 1:1). Because the ratio of P to QRS is usually consistent, A-flutter is often
regular in comparison to its irregular counterpart, A-fib. Atrial Flutter is also not necessarily a tachycardia
unless the AV node permits a ventricular response greater than 100 beats per minute.
AV nodal reentrant tachycardia (AVNRT) is also sometimes referred to as a junctional
reciprocating tachycardia. It involves a reentry circuit forming just next to or within the AV node itself. The
circuit most often involves two tiny pathways one faster than the other, within the AV node. Because the AV
node is immediately between the atria and the ventricle, the re-entry circuit often stimulates both, meaning
10
that a retrogradely conducted p-wave is buried within or occurs just after the regular, narrow QRS
complexes.
Atrioventricular reentrant tachycardia (AVRT) also results from a reentry circuit, although
one physically much larger than AVNRT. One portion of the circuit is usually the AV node, and the other, an
abnormal accessory pathway from the atria to the ventricle. Wolff-Parkinson-White syndrome is a relatively
common abnormality with an accessory pathway, the Bundle of Kent crossing the A-V valvular ring.
o
In orthodromic AVRT, atrial impulses are conducted down through the AV node and
retrogradely re-enter the atrium via the accessory pathway. A distinguishing characteristic of orthodromic
AVRT can therefore be a p-wave that follows each of its regular, narrow QRS complexes, due to retrograde
conduction.
o
In antidromic AVRT, atrial impulses are conducted down through the accessory pathway
and re-enter the atrium retrogradely via the AV node. Because the accessory pathway initiates conduction in
the ventricles ouside of the bundle of His, the QRS complex in antidromic AVRT is often wider than usual,
with a delta wave.
Finally, Junctional Ectopic Tachycardia or JET is a rare tachycardia caused by increased
automaticity of the AV node itself initiating frequent heart beats. On the EKG, junctional tachycardia often
presents with abnormal morphology p-waves that may fall anywhere in relation to a regular, narrow QRS
complex.
Differential Diagnosis
Atrial fibrillation, flutter
Sinus tachycardia
Reentry supraventricular tachycardias
Acute Treatment
In general, SVT is not life threatening, but episodes should be treated or prevented. While some
treatment modalities can be applied to all SVTs with impunity, there are specific therapies available to cure
some of the different sub-types. Cure requires intimate knowledge of how and where the arrhythmia is
initiated and propagated.
The SVTs can be separated into two groups, based on whether they involve the AV node for impulse
maintenance or not. Those that involve the AV node can be terminated by slowing conduction through the
AV node. Those that do not involve the AV node will not usually be stopped by AV nodal blocking
manoevres. These manoevres are still useful however, as transient AV block will often unmask the
underlying rhythm abnormality.
AV nodal blocking can be achieved in at least three different ways:
Physical maneuvers
A number of physical maneuvers cause increased AV nodal block, principally through activation of
the parasympathetic nervous system, conducted to the heart by the Vagus nerve. These manipulations are
therefore collectively referred to as vagal maneuver.
The best recognised of these is the Valsalva maneuver, which increases intra-thoracic pressure and
affects baro-receptors (pressure sensors) within the arch of the aorta. This can be achieved by asking the
patient to hold their breath and "bear down" as if straining to pass a bowel motion, or less embarrassingly, by
getting them to hold their nose and blow out against it. Plunging the face into, or just drinking a glass of ice
cold water is also often effective. Firmly pressing the bulb at the top of one of the carotid arteries in the neck
(carotis sinus massage, stimulating carotid baro-receptors) is also effective, but not recommended for those
without adequate medical training.
Drug Treatment
Another modality involves treatment with medications. Prehospital care providers and hospital
clinicians might administer Adenosine, an ultra short acting AV nodal blocking agent. If this works,
followup therapy with Diltiazem, Verapamil or Metoprolol may be indicated. SVT that does NOT involve
the AV node may respond to other anti-arrhythmic drugs such as Sotalol or Amiodarone.
In pregnancy, Metoprolol is the treatment of choice as recommended by the American Heart
Association.
Electrical Cardioversion
If physical maneuvers or drugs do not work, or if the patient is extremely unstable, a DC shock
delivered to the chest (synchronized cardioversion) may also be used, and is almost always effective.
Prevention & Cure
11
Once the acute episode has been terminated, ongoing treatment may be indicated to prevent a
recurrence of the arrhythmia. Patients who have a single isolated episode, or infrequent and minimally
symptomatic episodes usually do not warrant any treatment except observation.
Patients who have more frequent or disabling symptoms from their episodes generally warrant some
form of preventative therapy. A variety of drugs including simple AV nodal blocking agents like betablockers and verapamil, as well as anti-arrhythmics may be used, usually with good effect, although the risks
of these therapies need to be weighed against the potential benefits.
For supraventricular tachycardia caused by a re-entrant pathway, another form of treatment is
radiofrequency ablation. This is a low risk procedure that uses a catheter inside the heart to deliver
radiofrequency energy to locate and destroy the abnormal electrical pathways. Ablation has been shown to
be highly effective: up to 99% effective in eliminating AVNRT, and similar results in typical Atrial flutter.
Atrial flutter
Atrial flutter is an abnormal heart rhythm that occurs in the atria of the heart. When it first occurs, it
is usually associated with a fast heart rate or tachycardia, and falls into the category of supra-ventricular
tachycardias. While this rhythm occurs most often in individuals with cardiovascular disease (eg:
hypertension, coronary artery disease, and cardiomyopathy), it may occur spontaneously in people with
otherwise normal hearts. It is typically not a stable rhythm, and frequently degenerates into atrial fibrillation.
However, it does rarely persist for months to years.
Symptoms
While atrial flutter can sometimes go unnoticed, its onset is often marked by characteristic sensations
of regular palpitations. Such sensations usually last until the episode resolves, or until the heart rate is
controlled.
Atrial flutter is usually well tolerated initially (fast heart beat is for most people, just a normal
response to exercise), however, people with other underlying heart disease or poor exercise tolerance may
rapidly develop symptoms, which can include shortness of breath, chest pains, lightheadedness or dizziness,
nausea and, in some patients, nervousness and feelings of impending doom.
Prolonged fast flutter may lead to decompensation with loss of normal heart function (heart failure).
This may manifest as effort intolerance (exertional breathlessness), nocturnal breathlessness, or swelling of
the legs or abdomen.
Pathophysiology: mechanism of action
Atrial flutter is caused by a reentrant rhythm in either the right or left atrium. Typically initiated by a
premature electrical impulse arising in the atria, atrial flutter is propogated due to differences in refractory
periods of atrial tissue. This creates a self perpetuating loop of electrical activity moving around the atrium.
The impact and symptoms of atrial flutter depend on the heart rate of the patient. Heart rate is a
measure of the ventricular rather than atrial activity. Impulses from the atria are conducted to the ventricles
through the atrio-ventricular node. Due primarily to its longer refractory period, the AV node exerts a
protective effect on heart rate by blocking atrial impulses in excess of about 180 beats/minute (This block is
dependent on the age of the patient, and can be calculated roughly by subtracting patient age from 220). If
the flutter rate is 300/minute only half of these impulses will be conducted, giving a ventricular rate of
150/minute, ie. 2:1 block. The addition of rate-controlling drugs or conduction system disease can increase
this block substantially (see image below).
There are two types of atrial flutter, the common type I and rarer type II.1 Most individuals with
atrial flutter will manifest only one of these. Rarely someone may manifest both types; however, they can
only manifest one type at a time.
Type I atrial flutter, also known as common atrial flutter or typical atrial flutter, has an atrial rate
of 240 to 350 beats/minute. However, this rate may be slowed by antiarrhythmic agents.
The reentrant loop circles the right atrium, passing through the isthmus - a body of fibrous tissue in
the lower atrium between the inferior vena cava, and the tricuspid valve. Type I flutter is further divided into
two subtypes, known as counterclockwise atrial flutter and clockwise atrial flutter depending on the
direction of current passing through the loop. Counterclockwise atrial flutter (known as cephalad-directed
atrial flutter) is more commonly seen. The flutter waves in this rhythm are inverted in ecg leads II, III, and
aVF. The re-entry loop cycles in the opposite direction in clockwise atrial flutter, thus the flutter waves are
upright in II, III, and aVF.
Catheter ablation of the isthmus is a procedure usually available in the electrophysiology laboratory.
Eliminating conduction through the isthmus prevents reentry, and if successful, prevents the recurrence of
the atrial flutter.
12
Type II flutter follows a significantly different re-entry pathway to type I flutter, and is typically
faster, usually 340–430 beats/minute.
Treatment
In general, atrial flutter should be treated the same as atrial fibrillation. Because both rhythms can
lead to the formation of thrombus in the atria, individuals with atrial flutter usually require some form of
anticoagulation or anti-platelet agent. Both rhythms can be associated with dangerously fast heart rate and
thus require medication for rate and or rhythm control. Additionally, there are some specific considerations
particular to treatment of atrial flutter.
Cardioversion
Atrial flutter is considerably more sensitive to electrical direct-current cardioversion than atrial
fibrillation, and usually requires a lower energy shock. Conversely, it is relatively resistant to chemical
cardioversion, and often deteriorates into atrial fibrillation prior to spontaneous return to sinus rhythm.
Ablation
Because of the reentrant nature of atrial flutter, it is often possible to ablate the circuit that causes
atrial flutter. This is done in the electrophysiology lab by causing a ridge of scar tissue that crosses the path
of the circuit that causes atrial flutter. Ablation of the isthmus, as discussed above, is a common treatment for
typical atrial flutter.
Measurement of Successful Ablation
1.
Corridor of Widely split double potentials 90-110 ms
2.
Transisthmus Conduction Intervals
1.
Counter Clockwise defined as interval between stimulus on lateral wall and proximal
coronary sinus electrode.
2.
Clockwise defined as interval between stimulus in proximal CS and electrodes lateral to line
of block.
3.
Interval measured at 500, 400, and 300 ms. If this value increased by 50% or more this was
defined as successs or 150ms
3.
Pacing at multiple sites. AD>BD and DA>CA
4.
Bipolar electrograms lateral to line and pace from Proximal CS. Transition of polarity from
positive to negative
5.
3 pacing site protocol: Pace at two sites lateral (L1R and L2R) to the line on block and on the
septal site (S) of the line. Measure the conduction delay from the pacing site to the R wave on the QRS (L1
to R, L2 to R and S to R). If (L1R-L2R) > 0 and (L1R-SR) > 94 then there is a 100% sensitivity and 98%
specificity.
Electrocardiographic Findings
1.
There are rapid regular undulations (F waves) that cause a sawtooth appearance.
o
Best seen in 2,3,F and V1.
o
Usually inverted in the inferior leads.
o
No isoelectric baselines between the F waves.
2.
Atrial rate is 250 to 350 Beats Per Minute (BPM).
o
Can be faster in infants and children.
o
Massive dilation of the atria can lead to a rate < 200 BPM.
o
Quinidine can reduce the atrial rate.
3.
There is a variable ventricular rate depending on the AV conduction.
o
The Most common response is 2:1
o
3:1 is uncommon
o
4:1 suggests the existence of an AV conduction defect
o
May be associated with complete AV block in which case the RR intervals are regular and
the F waves have no constant relationship to the QRS. The ventricular response is usually slow.
o
1:1 conduction may be precipitated by excitement, exercise, induction of anesthesia or any
increase in sympathetic tone. It may occur in WPW where the impulses are conducted antegrade through the
bypass tract. All these are an emergency.
o
During treatment with quinidine the atrial rate may slow sufficiently to permit 1:1
conduction.
o
Vagal maneuvers increase the degree of AV block.
4.
QRS either normal or aberrant depending on preexisting IVCD or aberrant ventricular
conduction.
13
Complications
Although often regarded as a relatively benign rhythm problem, atrial flutter shares the same
complications as the related condition atrial fibrillation. There is paucity of published data directly
comparing the two, but overall mortality in these conditions appears to be very similar3.
Rate Related
Rapid heart rates may produce significant symptoms in patients with pre-existing heart disease. Even
in patients whose hearts are normal to start with, prolonged tachycardia tends to produce ventricular
decompensation and heart failure.
Clot formation
Because there is little if any effective contraction of the atria there is stasis (pooling) of blood in the
atria. Stasis of blood in susceptible individuals can lead to formation of thrombus (blood clots) within the
heart. Thrombus is most likely to form in the atrial appendages. Clot in the left atrial appendage is
particularly important since the left side of the heart supplies blood to the entire body. Thus, any thrombus
material that dislodges from the this side of the heart can embolize to the brain, with the potentially
devastating consequence of a stroke. Thrombus material can of course embolize to any other portion of the
body, though usually with a less severe outcome.
Sudden cardiac death
Sudden death is not directly associated with atrial flutter. However, in individuals with a pre-existing
accessory conduction pathway, such as the bundle of Kent in Wolff-Parkinson-White syndrome, the
accessory pathway may conduct activity from the atria to the ventricles at a rate that the AV node would
usually block. Bypassing the AV node, the atrial rate of 300 beats/minute leads to a ventricular rate of 300
beats/minute (1:1 conduction). Even if the ventricles are able to sustain a cardiac output at such a high rates,
1:1 flutter with time may degenerate into ventricular fibrillation, causing hemodynamic collapse and death.
Examples
Atrial
flutter
3:1
flutter
Atrial flutter
variable conduction
Atrial
4:1 Atrial flutter
A very rare condition
with1:1 Atrial flutter
2:1 Atrial flutter
Atrial
RBBB
flutter
with
Typical
flutter pattern
atrial
Atrial fibrillation etiology and differential diagnosis
Etiology of atrial fibrillation
AF can be associated with underlying cardiac diseases, but it may also occur in otherwise normal
hearts.
Common Causes
Hypertension
Heart failure
Coronary artery bypass surgery
Complete Differential Diagnosis of Underlying Etiologies for Atrial Fibrillation
Acute myocardial infarction • Congenital heart disease especially atrial septal defect in
adults • Coronary artery disease • Heart failure (especially diastolic dysfunction and
diastolic heart failure) • Hypertrophic cardiomyopathy (HCM) • Hypertension • Mitral
regurgitation Mitral stenosis (e.g. due to Rheumatic heart disease or Mitral valve
Cardiovascular
prolapse) • Myocarditis • Pericarditis • Previous heart surgery • Dual-chamber
pacemakers in the presence of normal atrioventricular conduction. • Restrictive
cardiomyopathies (such as amyloidosis, hemochromatosis, and endomyocardial
14
fibrosis), cardiac tumors, and constrictive pericarditis
Congenital
Dermatologic
No underlying causes
Drugs
Digoxin in patients with vagally mediated AF
Ear Nose Throat
Endocrine
No underlying causes
Hyperthyroidism • Hypothyroidism • Pheochromocytoma
Gastroenterologic
Vomiting
Genetic
A family history of AF increases risk by 30%. Various genetic mutations may be
responsible.
Hematologic
No underlying causes
Infectious Disease
No underlying causes
Musculoskeletal /
Ortho
No underlying causes
Neurologic
Nutritional /
Metabolic
Oncologic
Multiple sclerosis
Opthalmologic
No underlying causes
Overdose / Toxicity
Excessive alcohol consumption ("binge drinking" or "holiday heart syndrome") •
Carbon monoxide poisoning • Caffeine • Stimulants
Post-Op
Complication
Surgery,particularly coronary artery bypass surgery • During pulmonary artery line
placement and right heart catheterization trauma to the right atrium can result in atrial
fibrillation
Pulmonary
Hypoxia of any cause • Lung cancer • Pneumonia • Pulmonary embolism • Sarcoidosis
• sleep apnea syndrome
No underlying causes
No underlying causes
Renal / Electrolyte Hypokalemia
Rheum / Immune /
No underlying causes
Allergy
Electrocution • Cardiac contusion
Trauma
Hypothermia • Fever
Miscellaneous
The autonomic nervous system may trigger AF in susceptible patients through heightened
vagal or adrenergic tone
Morphology
The primary pathologic change seen in atrial fibrillation is the progressive fibrosis of the atria. This
fibrosis is primarily due to atrial dilatation, however genetic causes and inflammation may have a cause in
some individuals.
Dilatation of the atria can be due to almost any structural abnormality of the heart that can cause a
rise in the intra-cardiac pressures. This includes valvular heart disease (such as mitral stenosis, mitral
regurgitation, and tricuspid regurgitation), hypertension, and congestive heart failure. Any inflammatory
state that affects the heart can cause fibrosis of the atria. This is typically due to sarcoidosis but may also be
due to autoimmune disorders that create autoantibodies against myosin heavy chains. Mutation of the lamin
AC gene is also associated with fibrosis of the atria that can lead to atrial fibrillation.
Once dilatation of the atria has occurred, this begins a chain of events that leads to the activation of
the renin aldosterone angiotensin system (RAAS) and subsequent increase in matrix metaloproteinases and
disintegrin, which leads to atrial remodeling and fibrosis, with loss of atrial muscle mass.
This process is not immediate, and experimental studies have revealed patchy atrial fibrosis may
precede the occurrence of atrial fibrillation and may progress with prolonged durations of atrial fibrillation.
Fibrosis is not limited to the muscle mass of the atria, and may occur in the sinus node (SA node)
and atrioventricular node (AV node), correlating with sick sinus syndrome. Prolonged episodes of atrial
15
fibrillation have been shown to correlate with prolongation of the sinus node recovery time, suggesting that
dysfunction of the SA node is progressive with prolonged episodes of atrial fibrillation.
Signs and symptoms
In general, clinical manifestations are;
1.
Palpitations
2.
Chest pain
3.
Dyspnea
4.
Fatigue
5.
Lightheadedness
6.
Syncope: Syncope is an uncommon but serious complication that is usually associated with
sinus node dysfunction or hemodynamic obstruction, such as valvular aortic stenosis, HCM, cerebrovascular
disease, or an accessory AV pathway.
Atrial fibrillation is usually accompanied by symptoms related to the rapid heart rate. Rapid and
irregular heart rates may be perceived as palpitations, exercise intolerance, and occasionally produce angina
(if the rate is faster and puts the heart under strain) and congestive symptoms of shortness of breath or
edema. Sometimes the arrhythmia will be identified only with the onset of a stroke or a transient ischemic
attack (TIA, stroke symptoms resolving within 24 hours). It is not uncommon to identify atrial fibrillation on
a routine physical examination or electrocardiogram (ECG/EKG), as it may be asymptomatic in many cases.
As most cases of atrial fibrillation are secondary to other medical problems, the presence of chest
pain or angina, symptoms of hyperthyroidism (an overactive thyroid gland) such as weight loss and diarrhea,
and symptoms suggestive of lung disease would indicate an underlying cause. A previous history of stroke or
TIA, as well as hypertension (high blood pressure), diabetes, heart failure and rheumatic fever, may indicate
whether someone with atrial fibrillation is at a higher risk of complications.
Atrial fibrillation maintenance of sinus rhythm
ACC / AHA Guidelines- Maintenance of Sinus Rhythm (DO NOT EDIT)
Class I
1. Before initiating antiarrhythmic drug therapy, treatment of precipitating or reversible causes of AF
is recommended. (Level of Evidence: C)
Class IIa
1. Pharmacological therapy can be useful in patients with AF to maintain sinus rhythm and prevent
tachycardia induced cardiomyopathy. (Level of Evidence: C)
2. Infrequent, well-tolerated recurrence of AF is reasonable as a successful outcome of
antiarrhythmic drug therapy. (Level of Evidence: C)
3. Outpatient initiation of antiarrhythmic drug therapy is reasonable in patients with AF who have no
associated heart disease when the agent is well tolerated. (Level of Evidence: C)
4. In patients with lone AF without structural heart disease, initiation of propafenone or flecainide
can be beneficial on an outpatient basis in patients with paroxysmal AF who are in sinus rhythm at the time
of drug initiation. (Level of Evidence: B)
5. Sotalol can be beneficial in outpatients in sinus rhythm with little or no heart disease, prone to
paroxysmal AF, if the baseline uncorrected QT interval is less than 460 ms, serum electrolytes are normal,
and risk factors associated with class III drug–related pro-arrhythmia are not present. (Level of Evidence: C)
6. Catheter ablation is a reasonable alternative to pharmacological therapy to prevent recurrent AF in
symptomatic patients with little or no LA enlargement. (Level of Evidence: C)
Class III
1. Antiarrhythmic therapy with a particular drug is not recommended for maintenance of sinus
rhythm in patients with AF who have well-defined risk factors for proarrhythmia with that agent. (Level of
Evidence: A)
2. Pharmacological therapy is not recommended for maintenance of sinus rhythm in patients with
advanced sinus node disease or AV node dysfunction unless they have a functioning electronic cardiac
pacemaker. (Level of Evidence: C)
AV nodal reentrant tachycardia
AV nodal reentrant tachycardia (AVNRT) is a type of tachycardia (fast rhythm) of the heart. It is
a supraventricular tachycardia, meaning that it originates from a location within the heart above the bundle of
HIS. AV nodal reentrant tachycardia is the most common regular supraventricular tachycardia. It is more
common in women than men (approximately 75% of cases occurring in females). This tachycardia is
characterized by the sudden onset and sudden offset of rapid palpitations. AVNRT may be associated with
syncope, especially at the onset of the tachycardia. It is rarely life threatening.
16
AVNRT occurs when a reentry circuit forms within or just next to the atrioventricular node. The
circuit usually involves two anatomical pathways: the fast pathway and the slow pathway, which are both in
the right atrium. The slow pathway (which is usually targeted for ablation) is located inferiorly and slightly
posterior to the AV node, often following the anterior margin of the coronary sinus. The fast pathway is
usually located just superior and posterior to the AV node. These pathways are formed from tissue that
behaves very much like the AV node, and some authors regard them as part of the AV node.
Types
1.
AVNRT Slow/Fast
2.
AVNRT Fast/Slow
3.
AVNRT Slow/Slow
4.
AVNRT Slow/Fast Left Variant
There are several types of AVNRT. The "common form" or "usual" AVNRT utilizes the slow AV
nodal pathway as the anterograde limb of the circuit and the fast AV nodal pathway as the retrograde limb.
The reentry circuit can be reversed such that the fast AV nodal pathway is the anterograde limb and the slow
AV nodal pathway is the retrograde limb. This, not surprisingly is referred to as the "uncommon form" of
AVNRT. However, there is also a third type of AVNRT that utilizes the slow AV nodal pathway as the
anterograde limb and left atrial fibers that approach the AV node from the left side of the inter-atrial septum
as the retrograde limb. This is known as atypical, or Slow-Slow AVNRT.
Common AVNRT
In common AVNRT, the anterograde conduction is via the slow pathway and the retrograde
conduction is via the fast pathway ("slow-fast" AVNRT).
Because the retrograde conduction is via the fast pathway, stimulation of the atria (which produces
the inverted P wave) will occur at the same time as stimulation of the ventricles (which causes the QRS
complex). As a result, the inverted P waves may not be seen on the surface ECG since they are buried with
the QRS complexes. Often the retrograde p-wave is visible, but also in continuity with the QRS complex,
appearing as a "pseudo R prime" wave in lead V1 or a "pseudo S" wave in the inferior leads.
Uncommon AVNRT
In uncommon AVNRT, the anterograde conduction is via the fast pathway and the retrograde
conduction is via the slow pathway ("fast-slow" AVNRT). Multiple slow pathways can exist so that both
anterograde and retrograde conduction are over slow pathways. ("slow-slow" AVNRT).
Because the retrograde conduction is via the slow pathway, stimulation of the atria will be delayed
by the slow conduction tissue and will typically produce an inverted P wave that falls after the QRS complex
on the surface ECG.
Fast and slow pathways vs. accessory pathways
The fast and slow pathways should not be confused with the accessory pathways that give rise to
Wolff-Parkinson-White syndrome (WPW) syndrome or atrioventricular re-entrant tachycardia (AVRT).
In AVNRT, the fast and slow pathways are located within the right atrium in close proximity to or
within the AV node and exhibit electrophysiologic properties similar to AV nodal tissue.
Accessory pathways that give rise to WPW syndrome and AVRT are located in the atrioventricular
valvular rings, they provide a direct connection between the atria and ventricles, and have electrophysiologic
properties similar to ventricular myocardium.
Treatment
An episode of supraventricular tachycardia (SVT) due to AVNRT can be terminated by any action
that transiently blocks the AV node. This is because the AV node is an essential portion of the reentrant
circuit in AVNRT.
Medical therapy can be initiated with AV nodal slowing drugs such as adenosine, beta blockers or
calcium channel blockers. Increasing vagal tone, through measures such as carotid sinus massage, or the
valsalva maneuver, can sometimes terminate the tachycardia.
After being diagnosed with AVNRT, patients can also undergo an electrophysiology (EP) study to
confirm the diagnosis. Catheter ablation of the slow pathway, if successfully carried out, cures the patient of
AVNRT.
Wolff-Parkinson-White syndrome
Wolff-Parkinson-White syndrome (WPW) is a syndrome of pre-excitation of the ventricles of the
heart due to an accessory pathway known as the Bundle of Kent. This accessory pathway is an abnormal
electrical communication from the atria to the ventricles.
The incidence of WPW syndrome is between 0.1 and 3% of the general population.
17
While the vast majority of individuals with WPW syndrome remain asymptomatic throughout their
entire lives, there is a risk of sudden death associated with the syndrome. Sudden death due to WPW
syndrome is rare (incidence of less than 0.6%), and is due to the effect of the accessory pathway on
tachyarrhythmias in these individuals.
History
Described more than 50 yrs ago and named for John Parkinson, Paul Dudley White (Paul Dudley
White is a M.G.H. physician, evaluated a series of 11 healthy young patients who had attacks of paroxysmal
tachycardias in the presence of an EKG which showed a bundle branch block pattern with a short PR
interval), and Louis Wolff.
Pathophysiology
In normal individuals, electrical activity in the heart is initiated in the sinoatrial (SA) node (located in
the right atrium), propagates to the atrioventricular (AV) node, and then through the bundle of His to the
ventricles of the heart. (See electrical conduction system of the heart).
The AV node acts as a gatekeeper, limiting the electrical activity that reaches the ventricles of the
heart. This function of the AV node is important, because if the signals generated in the atria of the heart
were to increase in rate (as they do during atrial fibrillation or atrial flutter), the AV node will limit the
electrical activity that conducts to the ventricles. For instance, if the atria are electrically activated at 300
beats per minute, half those electrical impulses are blocked by the AV node, so that the ventricles are
activated at 150 beats per minute (giving a pulse of 150 beats per minute). Another important property of the
AV node is that it slows down individual electrical impulses. This is manifest on the ECG as the PR interval,
the time from activation of the atria (manifest as the P wave) and activation of the ventricles (manifest as the
QRS complex).
Individuals with WPW syndrome have an accessory pathway that connects the atria and the
ventricles, in addition to the AV node. This accessory pathway is known as the bundle of Kent. This
accessory pathway does not share the rate-slowing properties of the AV node, and may conduct electrical
activity at a significantly higher rate than the AV node. For instance, in the example above, if an individual
had an atrial rate of 300 beats per minute, the accessory bundle may conduct all the electrical impulses from
the atria to the ventricles, causing the ventricles to activate at 300 beats per minute. Extremely fast heart rates
are potentially dangerous, and can cause hemodynamic instability. In some cases, the combination of an
accessory pathway and cardiac arrhythmias can trigger ventricular fibrillation, a leading cause of sudden
cardiac death.
Diagnosis
WPW syndrome is commonly diagnosed on the basis of the surface ECG in an asymptomatic
individual. In this case it is manifested as a delta wave, which is a slurred upstroke in the QRS complex that
is associated with a short PR interval. The short PR interval and slurring of the QRS complex is actually the
impulse making it through to the ventricles prematurely (across the accessory pathway) without the usual
delay experienced in the AV node.
If the patient experiences episodes of atrial fibrillation, the ECG will show a rapid polymorphic
wide-complex tachycardia (without turning of the points). This combination of atrial fibrillation and WPW is
considered dangerous, and most antiarrhythmic drugs are contraindicated.
When an individual is in normal sinus rhythm, the ECG characteristics of WPW syndrome are a
short PR interval, widened QRS complex (greater than 120 ms in length) with slurred upstroke of the QRS
complex, and secondary repolarization changes reflected in ST segment-T wave changes.
In individuals with WPW syndrome, electrical activity that is initiated in the SA node travels through
the accessory pathway as well as through the AV node to activate the ventricles via both pathways. Since the
accessory pathway does not have the impulse slowing properties of the AV node, the electrical impulse first
activates the ventricles via the accessory pathway, and immediately afterwards via the AV node. This gives
the short PR interval and slurred upstroke to the QRS complex known as the delta wave.
Patients with WPW often exhibit more than one accessory pathway, and in some patients as many as
eight additional abnormal pathways can be found. This has been seen in individuals with Ebstein's anomaly.
Wolff-Parkinson-White syndrome is sometimes associated with Leber's hereditary optic neuropathy
(LHON), a form of mitochondrial disease.
18
One beat from a rhythm strip in V2 demonstrating characteristic findings in WPW syndrome. Note
the characteristic delta wave (subtler here than in some cases), the short PR interval of 0.08 seconds, and the
long QRS complex at 0.12 seconds.
The EKG in WPW
1.
Two pathways between the atrium and the ventricle are present.
2.
There is a shortened PR interval
o
PR less than 0.12 seconds
o
in most cases it varies between 0.08 and 0.11 seconds
3.
A wide QRS with a delta wave.
o
the QRS is 0.11 second or longer
o
is inversely proportional to the PR (i.e. the shorter the PR, the longer the QRS secondary to
greater pre-excitation).
o
the combination of the shortened PR interval and widened QRS is of normal duration
4.
The delta wave occurs as the ventricle is activated first via the accessory pathway (AP) and
then normal activation follows down the normal pathway.
o
the duration of the delta wave is 0.03 to 0.06 seconds
5.
The pattern of ventricular activation is determined by several factors:
o
the location of the accessory pathway: The closer the accessory pathway to the SA node, the
quicker the impulse will reach the atrial insertion site of the AP. In contrast, in those patients in whom the
AP is located in the far lateral region of the left ventricle, contribution to the AP during NSR may be
minimal.
o
the intra-atrial conduction time: Left atrial pathology will prolong the time necessary to
reach the left sided AP, drugs can also prolong the time to reach a left-sided pathway.
o
the conduction time over the accessory pathway: The conduction time over the AP depends
on the length of the AP and velocity with which the impulse is conducted. Investigators have found that the
accessory pathway may vary in length from 1 to 10 mm.
o
the AV conduction time over the normal AV nodal-His-Purkinje pathway
6.
Secondary T wave changes:
o
Because of the early asynchronous activation of the ventricle, the sequence of repolarization
will be different leading to T wave changes.
o
the T wave polarity is opposite in direction to the delta wave
7.
Concealed bypass tracts:
o
If the accessory pathway's contribution to ventricular activation is minimal because of the
coincidental arrival of the excitation wavefront over the normal pathway, then this should not be called a
concealed accessory pathway.
o
Concealed accessory pathways are those that conduct in a retrograde fashion
(ventriculoatrial) only.
o
Antegrade conduction in these patients is absent because the refractory period of the AP in
the antegrade direction is longer than the sinus cycle length.
o
when a recurrent tachycardia occurs in association with such concealed bypass, the
conduction is called concealed WPW syndrome
o
are usually located on the left side of the cardiac chambers
o
consider this if during the tachycardia there is a negative P wave in lead V1, if there is a P
wave after the QRS complex
8.
Findings are intermittent in 1/2 the cases
19
Wolf Parkinson White Anterolateral
Pathway
Wolf Parkinson White Anteroseptal
Pathway
Wolf Parkinson White
Wolf Parkinson White Anteroseptal
Epicardial
Pathway
Wolf Parkinson White Syndrome Posteroseptal
Wolf Parkinson White Left Posterior
Pathway
Pathway
Incidence
1.
Somewhere between 0.1 and 3 per 1000 EKGs.
2.
May be underdiagnosed given the presence of left sided APs which may be silent during
NSR.
3.
The incidence of tachyarrhythmias in patients with WPW has been estimated to be
somewhere between 12% and 80%.
4.
WPW may be the most prevalent cause of paroxysmal regular supraventricular tachycardia
One series found that 57% of patients c psvt were subsequently found to have WPW syndrome on the resting
NSR EKG. If the attack occurs before the age of 21, then 73% of the patients had WPW. In patients > 21
years old, then WPW syndrome was found in 48% of patients with PSVT. (Wellens et al 1981).
EKG Classification
1.
Type A:
o
Prominent R wave in lead V1 and V2.
o
It has been found at EP studies that these patients have early activation of the left ventricle.
o
Generally V1 shows either a notched R wave or RS or Rsr' deflection
o
Mimics a posterior MI, RVH
2.
Type B:
o
Prominent S wave deflection in the right precordial leads, and upright R waves in the lateral
precordial leads.
20
o
EP studies have showed that this form of WPW syndromes is due to early activation of the
lateral aspect of the right ventricle
o
This form is more common.
o
May resemble an abnormal Q wave in the right precordial leads and be mistaken for an
anterior MI
o
In both type A and B there may be abnormal q waves in leads 2, 3 and aVF.
Determining the location of the accessory pathway
Check lead V1
negative delta wave in V1 = right ventricle
positive delta wave om V1= left ventricle
Negative delta wave and
Negative delta wave and isoelectric or negative
Left axis Inferior axis
QRS in II, III, AVF
QRS in II, III, AVF
delta I, AVL, V5, V6
Right
Posteroseptal
Anteroseptal Posteroseptal
Lateral
free wall
Oversimplification
Histological studies have found that the AP fibers may insert in the septum and not the free wall as
above. The location of AP may be impossible to determine in NSR, as it can be complicated by the existence
of more than one AP in some patients, the coexistence of congenital lesions, the occasional superimposition
of the P wave on the initial portion of the delta wave, and differences in the activation depending on whether
the AP is epicardially or endocardially located.
Associated Cardiovascular Abnormalities
1.
Type B is found in 5% to 25% of the reported cases of Ebstein's dz. Suspect this if there is
Type B WPW with RBBB.
2.
Also been found in patients with corrected transposition of the great arteries, tricuspid
atresia, endocardial fibroelastosis, MVP, cardiomyopathies (hypertrophic obstructive and congestive).
Clinical Manifestations
1.
The most common form of paroxysmal tachycardia in these patients is a circus movement
tachycardia (CMT) incorporating the AP.
2.
The CMT utilizes the following structures: the AV node, the His-Purkinje system, the
ventricular myocardium (from the terminal portion of the His system to the ventricular end of the AP), the
AP itself, and the atrial myocardium itself from the atrial insertion of the AP to the AV node.
3.
This circuit can conduct in both directions:
o
Type I A CMT:
1.
This is the usual form of the CMT in patients with WPW.
2.
Is antegrade through the AV node, VA conduction through the AP.
3.
The QRS complex during the tachycardia shows either normal intraventricular
conduction or typical bundle branch block configuration.
4.
Sx: Palpitations (97%), dyspnea (57%), anginal pain (56%), perspiration (55%),
fatigue (41%), anxiety (30%), dizziness (30%),polyuria (26%).
5.
this is also called orthodromic reentrant tachycardia
6.
there is no delta wave
7.
the rate is 140 to 250 bpm
8.
it is faster than the rate of tachycardia due to reentry in the AV node
9.
often triggered by a PAC
o
Type I B CMT:
1.
anterograde down the accessory pathway, retrograde down the AVN-His pathway.
2.
the QRS is widened
3.
this form is rarer
o
Type II CMT (intra-AV nodal):
1.
anterograde pathway is an AV nodal slow pathway, the retrograde pathway is an AV
nodal fast pathway.
2.
No evidence of ventricular pre-excitation during the tachycardia.
o
Type III CMT (uses two accessory pathways):
1.
Conducts anterograde down one accessory pathway and retrograde up a second
accessory pathway.
2.
These patients can also experience atrial tachycardias and ventricular tachycardias.
Atrial Fibrillation in WPW
21
Can cause life-threatening ventricular rates due to the exclusive AV conduction over the accessory
pathway.
1.
o
reduces cardiac output.
o
may degenerate into VF, particularly in those with multiple bypass tracts.
2.
The only marker identified for degeneration into VF in the literature was the occurrence of
RR intervals equal to or less than 205 msec during the a fib
3.
Seen in 78 of 256 of Wellen's patients with WPW. Reported incidence is 20 to 35% in other
studies.
4.
The degree of ventricular preexcitation observed in the EKG during NSR bears no
relationship whatsoever to the risk of developing life-threatening ventricular rates during the a.fib.
5.
The QRS complexes are wide and bizarre as a result of preexcitation.
6.
The ventricular rate is 220 to 360 beats per minute due to the short effective refractory
period of the accessory pathway.
7.
It is often mistaken for VT.
8.
If the atrial rate in atrial fibrillation is greater than 200 BPM then suspect this. The rhythm
will also be grossly irregular if it is due to atrial fibrillation. Such a rapid rate would be unusual if it were due
to conduction by way of the normal AV conduction system.
The upstroke of the QRS-complex is 'slurred',
resulting in a delta-wave (arrow).
Louis Wolff, Sir John Parkinson and Paul Dudley, who
discovered the phenomenon that later would be called the
WPW syndrome.
Delta waves in a patient with WolffA atrioventricular tachycardia through the accessory bundle. TheParkinson-White Syndrome (WPW)
electrical signal travels from the ventricles via the accessory bundle
to the atria and returns to the ventricles via the AV node.
WPW on a 12 lead
ECG.
Another example of WPW on a 12
lead ECG.
22
12 lead EKG: Wolff Parkinson White
12 lead EKG: Wolff Parkinson White Syndrome Type I. Courtesy
Syndrome
of Dr Jose Ganseman
12 lead EKG: Wolff Parkinson White Syndrome Type12 lead EKG: Wolff Parkinson White Syndrome Type
I. Courtesy of Dr Jose Ganseman
I. Courtesy of Dr Jose Ganseman
12 lead EKG: Wolff Parkinson White Syndrome Type12 lead EKG: Wolff Parkinson White Syndrome Type
II. Courtesy of Dr Jose Ganseman
II. Courtesy of Dr Jose Ganseman
12 lead EKG: Wolff Parkinson12 lead EKG: Wolff Parkinson12 lead EKG: Wolff Parkinson
White Syndrome Type II. CourtesyWhite Syndrome Type II. CourtesyWhite Syndrome Type II. Courtesy
of Dr Jose Ganseman
of Dr Jose Ganseman
of Dr Jose Ganseman
WPW syndrome with an orthodromic circus movement
tachycardia: Narrow complex tachycardia with a rate of 200 bpmThe same patient's EKG during sinus
(RR interval 320 ms). After 5 cycles, the tachycardia suddenlyrhythm. A discrete Δ wave is clearly
stops and four multiform complexes are seen without any Pvisible. The morphology of the Δ wave
waves. These complexes should be regarded as a polymorphicsuggests a left posterior Kent bundle.
ventricular tachycardia, which is not uncommon after an
adenosine-terminated supraventricular tachycardia. A 5th
complex is preceded by a P wave. The subsequent 4 complexes
23
show a widened QRS complex and all are immediately preceded
by a P wave. The initial phase of the QRS complex is slurred and
positive in all available leads. Sinus rhythm continues thereafter
with gradual abbreviation of the QRS complex until a 120 msec
wide QRS complex remains.
Electrophysiologic Studies in WPW
The purpose of these studies is to:
determine the location and number of accessory pathways.
determine the mechanism and pathway of the tachycardia.
to establish the diagnosis in those patients c a questionable resting EKG.
to evaluate the efficacy of drugs.
to select patients for surgery and to evaluate them postoperatively.
Risk stratification
12 lead EKG of an individual with WPW syndrome. The accessory pathway is located in the left
posteroseptal region.
Treatment is based on risk stratification of the individual. Risk stratification is performed to
determine which individuals with WPW syndrome are at risk for sudden cardiac death (SCD). Sudden
cardiac death in these individuals is due to the propagation of an atrial arrhythmia to the ventricles at a very
high rate.
A good history should be taken to determine whether an individual has factors suggestive of a
previous episode of unexplained syncope (fainting) or palpitations (sudden awareness of one's own, usually
irregular, heartbeat). These may be due to earlier episodes of a tachycardia associated with the accessory
pathway.
Individuals with WPW syndrome in whom the delta waves disappear with increases in the heart rate
are considered at lower risk of SCD. This is because the loss of the delta wave shows that the accessory
pathway cannot conduct electrical impulses at a high rate (in the anterograde direction). These individuals
will typically not have fast conduction down the accessory pathway during episodes of atrial fibrillation.
Risk stratification is best performed via programmed electrical stimulation (PES) in the cardiac
electrophysiology lab. This is an invasive procedure, in which the rate of impulse propagation via the
accessory pathway is determined by stimulating the atria and by inducing transient atrial fibrillation.
High risk features that may be present during PES include an effective refractory period of the
accessory pathway less than 270 ms, multiple pathways, septal location of pathway, and inducibility of
supraventricular tachycardia. Individuals with any of these high risk features are generally considered at
increased risk for SCD and should be treated accordingly.
It is unclear whether invasive risk stratification (with programmed electrical stimulation) is
necessary in the asymptomatic individual. While some groups advocate PES for risk stratification in all
individuals under 35 years old, others only offer it to individuals who have history suggestive of a
tachyarrhythmia, since the incidence of sudden death is so low.
Treatment
Acutely, people with WPW who are experiencing a tachydysrhythmia may require electrical
cardioversion if their condition is critical, or, if more stable, medical treatment may be used. Patients with
atrial fibrillation and rapid ventricular response are often treated with amiodarone or procainamide to
stabilize their heart rate. Adenosine and other AV node blockers should be avoided in Atrial fibriliiatin with
WPW; this inlcudes adenosine, diltiazem, verapamil,other calcium channel blockers and Beta-blockers.
Patients with a rapid heart beat with narrow QRS complexes (circus movement tachycardias) may also be
cardioverted, alternatively, adenosine may be administered if equipment for cardioversion is immediately
available as a backup.
The definitive treatment of WPW syndrome is a destruction of the abnormal electrical pathway by
radiofrequency catheter ablation. This procedure is performed almost exclusively by cardiac
electrophysiologists. Radiofrequency catheter ablation is not performed in all individuals with WPW
24
syndrome because there are inherent risks involved in the procedure. Adeosine is contraindicated for patients
in atrial fibrillation or atrial flutter with a history of WPW
When performed by an experienced electrophysiologist, radiofrequency ablation has a high success
rate. If radiofrequency catheter ablation is successfully performed, the patient is generally considered cured.
Recurrence rates are typically less than 5% after a successful ablation. The one caveat is that individuals with
underlying Ebstein's anomaly may develop additional accessory pathways during progression of their
disease.
Circus Movement Tachycardias
Rapid heart rate is most likely due to CMT or atrial fibrillation.
Palpitations are usually regular during a CMT, irregularly with atrial fib.
Some patients report that their attacks of CMT may be broken by vagal maneuvers.
A 24 hr holter should be done in those patients with a suspicion of WPW to assess the mode
of initiation, and to determine the type (CMT vs afib).
If the patients quality of life is affected and the holter is negative, then EP studies should be
done.
If there is an inability to induce CMT at EP then it is very unlikely that this is the arrhythmia
that the patient is experiencing outside the hospital. Inability to induce CMT obviously does not exclude that
the patient is experiencing afib.
If the patient presents in a rapid rhythm, you should first try vagal maneuvers to terminate a
CMT.
The most likely arrhythmia on a statistical basis is a CMT with the AP conducting in a
retrograde fashion. If the AP is incorporated in a retrograde fashion then there are often retrograde P waves
apparent following the QRS, with a PR interval longer than the RP'interval.
If the patient is known to have WPW and has a regular tachycardia thought to be a CMT,
then verapamil should be the first drug used. If verapamil is not available, then use propranolol IV. Both
terminate the CMT by prolonging the refractory period at the AV node.
Digitalis has been used, however some patients may dev afib with this, and dig may
abbreviate the refractory period of the AP resulting in higher ventricular rates during the afib. Therefore the
use of dig is not recommended.
If drugs affecting the AV node are not effective, then drugs that affect the accessory pathway
are used such as procainamide or disopyramide.
If the above fail to terminate the tachycardia or if the patient is tolerating the tachycardia
poorly, then the patient should be paced out of it or cardioverted. Pacing is safer in patients on multiple
drugs.
Atrial Fibrillation
1.
Patients can experience high rates during afib because of conduction over the accessory
pathway which can have a very short refractory period.
2.
Mean ventricular rates in these patients range from 160 to 300 BPM.
3.
During these attacks there is not only the risk of hemodynamic compromise but also a risk of
degenerate into VF.
4.
As a rule dig should be avoided in these patients.
5.
Cardioversion is the tx of choice. If the patient is receiving drugs that promote asystole
following electrical cardioversion (e.g. verapamil, beta-blockers, and probably amiodarone) then a temporary
pacer should be positioned in the RV before the cardioversion.
6.
If the ventricular rate during the afib is not > 200, then you could try procainamide,
disopyramide, or quinidine which may prolong the refractory period of the accessory pathway.
Drug Prophylaxis In The Patient With Proven Tachyarrhythmias
1.
Should receive prophylactic treatment if the arrhythmia is poorly tolerated.
2.
In Wellen's experience amiodarone is the most effective in preventing attacks of paroxysmal
tachycardia in patients with WPW. Otherwise he recommends quinidine, disopyramide, procainamide or
propranolol alone or in combination.
3.
For those patients with life-threatening rates during afib, Wellen's recommends amiodarone
as prophylaxis which prolongs the AP refractory period. Quinidine is an alternative, but is less effective.
4.
These patients should undergo EP studies to assess the adequacy of treatment.
5.
These authors state that the refractory period of the AP can shorten in the presence of
sympathetic stimulation and advocate the addition of a beta blocker.
Approach to the Patient with a Questionable EKG
25
1.
As mentioned previously, the diagnosis can be difficult in those patients c normal EKGs at
2.
In some patients the diagnosis can be made with the following noninvasive procedures:
CSP to increase the AV nodal delay therefore enhancing conduction over the accessory
rest.
o
pathway.
o
IV procainamide may cause QRS abnormality to disappear by prolonging conduction down
the AP.
o
β-blockers, verapamil, digoxin may also facilitate the conduction down the accessory
pathway
Differential Diagnosis
Diagnosis of Hypertrophy, Bundle Branch Block and MI in the Presence of WPW are all obscured.
1.
Type A WPW
o
RBBB
o
RVH
o
True posterior MI
o
IMI
2.
Type B WPW
o
LBBB
o
Anterior MI
o
IMI
3.
WPW and atrial flutter
o
paroxysmal VT or flutter
Intractable Tachyarrhythmias in WPW
In patients with afib with rapid ventricular response then surgical interruption should be considered.
Variants of WPW
1.
LGL: Lown-Ganong-Levine Syndrome
o
there is a short PR, but no delta wave
o
due to intranodal bypass tracts (i.e. there is conduction down James fibers)
o
normal QRS duration
o
PR less than 0.12 seconds
o
normal P wave
2.
Mahaim Type of Preexcitation
o
nodoventricular, nodofascicular or fasciculoventricular connections
o
the impulse may travel through the AV node normally and this may then be followed by
premature conduction to the basal ventricular myocardium
o
there is a delta wave with a normal PR interval
o
is rarer than WPW or LGL
o
in older patients there can be a prolonged conduction down the accessory pathway resulting
in a normal PR interval in the presence of WPW which is tough to distinguish from Mahaim fibers
Figure : The same patient after Mahaim
Figure : A 24 years old man with Mahaim type of
bundle ablation
preexcitation
Ventricular tachycardia
Ventricular tachycardia (V-tach or VT) is a tachycardia, or fast heart rhythm that originates in one
of the ventricles of the heart. This is a potentially life-threatening arrhythmia because it may lead to
ventricular fibrillation and sudden death.
Classification
1. Classification Based Upon Morphology of Complexes
Ventricular tachycardia can be classified based on its morphology: Monomorphic ventricular
tachycardia means that the appearance of all the beats match each other in each lead of a surface
electrocardiogram (EKG).
26
Polymorphic ventricular tachycardia, on the other hand, has beat-to-beat variations in morphology.
This most commonly appears as a cyclical progressive change in cardiac axis referred to by its French
eponym Torsade de pointes (literally twisting of the points).
12 lead electrocardiogram showing a run of monomorphic ventricular tachycardia (VT)
2. Classification Based Upon Duration of Episode
Another way to classify ventricular tachycardias is the duration of the episodes: Technically, three
or more beats in a row on an EKG that originate from the ventricle at a rate of more than 100 beats per
minute constitute a ventricular tachycardia. If the fast rhythm self-terminates within 30 seconds, it is
considered a non-sustained ventricular tachycardia. If the rhythm lasts more than 30 seconds it is known
as a sustained ventricular tachycardia (even if it terminates on its own after 30 seconds).
Sustained Ventricular Tachycardia
Ventricular tachycardia originates from a ventricular focus
Lasts more than 30 seconds
Broad QRS complexes: rate of >90 beats/minute
Paroxysmal Ventricular Tachycardia
1.
Rapid succession of three or more ectopic beats.
2.
Sustained if it lasts longer than 30 seconds.
Incessant Ventricular Tachycardia
1.
The ventricular tachycardia is recurrent and the episodes are interrupted by only a few sinus
beats.
3. Classification Based Upon Symptoms
A third way to classify ventricular tachycardia is on the basis of its symptoms: Pulseless VT is
associated with no effective cardiac output, hence, no effective pulse, and is a cause of cardiac arrest. In this
circumstance it is best treated the same way as ventricular fibrillation (VF) and is recognized as one of the
shockable rhythms on the cardiac arrest protocol. Some VT is associated with reasonable cardiac output and
may even be asymptomatic. The heart usually tolerates this rhythm poorly in the medium to long term, and
patients may certainly deteriorate to pulseless VT or to VF.
Pathophysiology
The morphology of the tachycardia depends on its cause.
In monomorphic ventricular tachycardia, the reason all the beats look the same is because the
impulse is being generated from either increased automaticity of a single point in either the left or right
ventricle, or due to a reentry circuit within the ventricle. The most common cause of monomorphic
ventricular tachycardia is damaged or dead (scar) tissue from a previous myocardial infarction (heart attack).
This scar cannot conduct electrical activity, so there is a potential circuit around the scar that results in the
tachycardia. This is similar to the re-entrant circuits that are the cause of atrial flutter and the re-entrant forms
of supraventricular tachycardia. Other rarer congenital causes of monomorphic VT include right ventricular
dysplasia, and right and left ventricular outflow tract VT.
Polymorphic ventricular tachycardia, on the other hand, is most commonly caused by abnormalities
of ventricular muscle repolarization. The predisposition to this problem usually manifests on the EKG as a
prolongation of the QT interval. QT prolongation may be congenital or acquired. Congenital problems
include Long QT syndrome and Catecholaminergic polymorphic ventricular tachycardia. Acquired problems
are usually related to drug toxicity or electrolyte abnormalities, but can occur as a result of myocardial
ischaemia. Class III anti-arrhythmic drugs such as sotalol and amiodarone prolong the QT interval and may
in some circumstances be pro-arrhythmic. Other relatively common drugs including some antibiotics and
antihistamines may also be a danger, particularly in combination with one another. Problems with blood
levels of potassium, magnesium and calcium may also contribute. High dose magnesium is often used as an
antidote in cardiac arrest protocols.
27
Monomorphic ventricular tachycardia
Diagnosis
The diagnosis of ventricular tachycardia is made based on the rhythm seen on either a 12 lead
EKG or a telemetry rhythm strip. It may be very difficult to differentiate between ventricular tachycardia
and a wide-complex supraventricular tachycardia in some cases. In particular, supraventricular tachycardias
with aberrant conduction from pre-existing bundle branch block are commonly misdiagnosed as ventricular
tachycardia. Other rarer phenomena include ashman beats and antedromic atrioventricular re-entry
tachcyardias.
Various diagnostic criteria have been developed to determine if a wide complex tachycardia is
ventricular tachycardia or a more benign rhythm. In addition to these diagnostic criteria, if the individual has
a past history of a myocardial infarction, congestive heart failure, or recent angina, the wide complex
tachycardia is much more likely to be ventricular tachycardia.
The proper diagnosis is important, as the misdiagnosis of supraventricular tachycardia when
ventricular tachycardia is present is associated with worse prognosis. This is particularly true if calcium
channel blockers, such as verapamil are used to attempt to terminate a presumed supraventricular
tachycardia. It is therefore wisest to assume that all wide complex tachycardia is VT until proven otherwise.
EKG Findings
Ventricular tachycardia in Lead II (rhythm)
1.
Abnormal and wide QRS complexes with secondary ST segment and T wave changes.
o
Usual QRS duration is > 0.12 seconds, may be shorter if the ectopic focus is located in the
ventricular septum.
o
The secondary ST segment and T wave changes are in a direction that is opposite the major
deflection of the QRS.
o
A ventricular rate between 140 and 200 BPM.
o
When the rate is >200 and has a sine wave appearance, it is called ventricular flutter.
o
When the rate is <110 BPM it is called non-paroxysmal VT.
2.
A regular or slightly irregular (up to 0.03 seconds) rhythm.
3.
Abrupt onset and termination.
4.
AV dissociation.
o
Atrial rate slower than ventricular rate.
o
No relationship between atrial activity and ventricular activity.
o
There can be VA conduction.
1.
The RP interval is >0.11 seconds.
2.
Occurs in about 50% of cases.
3.
Uncommon when the ventricular rate is rapid (only 1/7 when the rate was>200).
5.
Capture beats.
o
Occurs when a supraventricular impulse is conducted and captures the ventricle.
o
They are rare.
6.
Fusion beats.
o
Rare in VT at a rapid rate.
28
Examples of Ventricular Tachycardia:
12
lead
tachycardia.
EKG:
Ventricular12 lead EKG: Ventricular tachycardia. Image courtesy of Dr Jose
Ganseman
12 lead EKG: Ventricular tachycardia. Image courtesy12 lead EKG: Ventricular tachycardia. Image courtesy
of Dr Jose Ganseman
of Dr Jose Ganseman
12 lead EKG: Ventricular tachycardia. Image courtesy12 lead EKG: Ventricular tachycardia. Image courtesy
of Dr Jose Ganseman
of Dr Jose Ganseman
Ventricular tachycardia of 140 bpm with a left bundleVentricular tachycardia of 250 bpm with a right bundle
branch block pattern and left heart axis.
branch block pattern and right heart axis.
Ventricular tachycardia of 150 bpm with a right bundle branch block patternVentricular flutter on a 12 lead
and right heart axis. Note the 5th and 6th complex from the right side. TheseECG
are fusion complexes.
Differential Diagnosis of Underlying Causes of Ventricular Tachycardia
Caffeine
Cardiomyopathy
Cocaine
Congenital Heart Disease
29
Congestive heart failure
Electrolyte imbalance
Hypertrophic cardiomyopathy
Hypocalcemia
Hypokalemia
Hypomagnesia
Iatrogenic due to pulmonary artery catheter, right heart catheterization, or electrophysiologic
sutdy
Methamphetamine
STEMI
Ventricular aneurysm
Treatment
Therapy may be directed at either terminating an episode of the arrhythmia or for suppressing a
future episode from occurring. The treatment is tailored to the specific patient, with regard to how well the
individual tolerates episodes of ventricular tachycardia, how frequently episodes occur, their comorbidities,
and their wishes.
Electrical Cardioversion / Defibrillation
It is usually possible to terminate a VT episode with a direct current shock across the heart. This is
ideally synchronised to the patient's heartbeat. As it is quite uncomfortable, shocks should be delivered only
to an unconscious or sedated patient. A patient with pulseless VT will be unconscious and treated as an
emergency on a cardiac arrest protocol. Elective cardioversion is usually performed in controlled
circumstances with anaesthetic and airway support.
The shock may be delivered to the outside of the chest using an external defibrillator, or internally to
the heart by an implantable cardioverter-defibrillator (ICD) if one has previously been inserted.
An ICD may also be set to attempt to overdrive pace the ventricle. Pacing the ventricle at a rate
faster than the underlying tachycardia can sometimes be effective in terminating the rhythm. If this fails after
a short trial, the ICD will usually stop pacing, charge up and deliver a defibrillation grade shock.
Antiarrhythmic drug therapy
Drugs such as amiodarone, epinephrine and vasopressin may be used in addition to [defibrillation] to
terminate VT while the underlying cause of the VT can be determined. Possible causes or contributing
factors to VT can be remembered as the six H's and five T's: Hypovolemia, Hypoxia, Hydrogen ion
(acidosis), Hypo- or Hyperglycemia, Hypothermia; and Toxins, Tamponade (cardiac), Tension
pneumothorax, Thrombosis, Trauma.
Long term anti-arrhythmic therapy may be indicated to prevent recurrence of VT. Beta-blockers and
a number of class III anti-arrhythmics are commonly used.
For some of the rare congenital syndromes of VT, other drugs, and sometimes even catheter ablation
therapy may be useful.
The implantation of an ICD is more effective than drug therapy for prevention of sudden cardiac
death due to VT and VF, but may be constrained by cost issues, and well as patient co-morbidities and
patient preference.
Bundle Branch Reentry Ventricular Tachycardia
Characteristics
Bundle Branch Reentry Ventricular Tachycardia usually occurs either in patients with structural
heart disease or in patients with conduction disturbances with a structurally normal heart. Bundle branch
reentry is a macro-reentrant tachycardia that incorporates the His-Purkinje system, the bundle branches, and
transseptal myocardial conduction in the circuit. Typical Bundle Branch reentry tachycardia uses the right
bundle as the anterograde limb and the left bundle as the retrograde limb. Atypical Bundle Branch reentry
30
uses the left bundle (anterior fascicle, posterior fascicle or both) as the antegrade limb and the right bundle as
the retrograde limb. The tachycardia appears as a typical Left Bundle Branch Block or Right Bundle Branch
Block respectively.
Diagnosis
Each ventricular activation is preceeded by His bundle activation (see Figure)
Changes in the HH Interval precede changes in the VV interval
Tachycardia induction is associated with critical delay in the HPS Initiation or a V-H
increment (“V-H jump”) (see Figure)
A longer H-V interval during tachycardia as compared with sinus rhythm. This is
paradoxical because one would expect it to be analogous to the situation with fascicular tachycardia, the
wavefront in Bundle Branch Reentry Ventricular Tachycardia usually proceeds retrogradely through the
left bundle branch system. It then divides with a portion continuing retrogradely to the site of the His bundle
recording, and another portion turning anterogradely along the right bundle branch to the ventricle. Since this
impulse enters the distal His bundle, and then proceeds retrogradely to the His bundle recording site and
simultaneously anterogradely down the right bundle branch, one might expect the ventricle to be depolarized
with an HV interval shorter than during sinus rhythm. This does not occur and the longer HV interval
typically seen in BBRT is probably due to a combination of some effects of relative refractoriness of the
right bundle and a predominant role of anisotropic conduction.
Tachycardia is terminated by block in the HPS
Tachycardia is abolished by ablation of the RBBB
Brugada syndrome
The Brugada syndrome is a genetic disease that is characterized by abnormal electrocardiogram
(ECG) findings and an increased risk of sudden cardiac death in young adults, and occasionally in children
and infants. It is also known as Sudden Unexpected Death Syndrome (SUDS), and is the most common
cause of sudden death in young men without known underlying cardiac disease in Thailand and Laos.
ECG pattern in Brugada syndrome. According to a recent consensus document, type 1 ST segment
elevation either spontaneously present or induced with Ajmaline/Flecainide test is considered diagnostic.
Type 1 and 2 may lead to suspicion but drug challenge is required for diagnosis. The ECGs in the right and
left panels are from the same patient before (right panel, type 1) and after (left panel, type 1) endovenous
administration of 1 mg/kg of Ajmaline during 10 minutes.
Although the ECG findings of Brugada syndrome were first reported among survivors of cardiac
arrest in 1989, it was only in 1992 that the Brugada brothers recognized it as a distinct clinical entity, causing
sudden death by causing ventricular fibrillation (a lethal arrhythmia) in the heart.
Differential Diagnosis
Abnormalities that can lead to ST-segment elevation in the right precordial leads
Acute myocardial ischemia or infarction
Acute myocarditis
Acute pulmonary thromboemboli
Arrhythmogenic right ventricular dysplasia / cardiomyopathy (ARVD/C)
Cocaine intoxication
Dissecting aortic aneurysm
Duchenne muscular dystrophy
Friedreich ataxia
31
Heterocyclic antidepressant overdose
Hypercalcemia
Hyperkalemia
Hypothermia, causing Osborn wave in ECGs and sometimes resembling Brugada syndrome
Left ventricular hypertrophy
Mediastinal tumor compressing the right ventricular outflow tract (RVOT)
Right or left bundle-branch block
Right ventricular infarction
Right ventricular ischemia
Thiamine deficiency
Various central and autonomic nervous system abnormalities
Other conditions that can lead to ST-segment elevation in the right precordial leads
Early repolarization syndrome
Other normal variants (particularly in males)
Epidemiology
The average age at the time of initial diagnosis or sudden death is 40 ± 22 years, with the youngest
patient diagnosed at 2 days of age and the oldest at 84 years. The prevalence of the Brugada syndrome is
estimated at 1–5 per 10,000 inhabitants worldwide. The frequency is lower in western countries and higher
(≥5 per 10,000) in Southeast Asia.
Genetics and pathophysiology
Approximately 20% of the cases of Brugada syndrome have been shown to be associated with
mutation(s) in the gene that encodes for the sodium ion channel in the cell membranes of the muscle cells of
the heart (the myocytes). The gene, named SCN5A, is located on the short arm of the third chromosome
(3p21). Loss-of-function mutations in this gene lead to a loss of the action potential dome of some epicardial
areas of the right ventricle. This results in transmural and epicardial dispersion of repolarization. The
transmural dispersion underlies ST-segment elevation and the development of a vulnerable window across
the ventricular wall, whereas the epicardial dispersion of repolarization facilitates the development of phase
2 reentry, which generates a phase 2 reentrant extrasystole that captures the vulnerable window to precipitate
ventricular tachycardia and/or fibrillation that often results in sudden cardiac death. At present time however,
all the reported patients died because of the disease and submitted to detailed necropsy study, have shown a
structural right ventricular pathology underlying the syndrome.
Electrocardiography
In some cases, the disease can be detected by observing characteristic patterns on an
electrocardiogram, which may be present all the time, or might be elicited by the administration of particular
drugs (e.g., Class IC antiarrythmic drugs that blocks sodium channels and causing appearance of ECG
abnormalities - ajmaline, flecainide) or resurface spontaneously due to as yet unclarified triggers. The pattern
seen on the ECG is persistent ST elevations in the electrocardiographic leadsV 1-V3 with a right bundle
branch block (RBBB) appearance with or without the terminal S waves in the lateral leads that are associated
with a typical RBBB. A prolongation of the PR interval (a conduction disturbance in the heart) is also
frequently seen.The electrocardiogram can fluctuate over time, depending on the autonomic balance and the
administration of antiarrhythmic drugs. Adrenergic stimulation decreases the ST segment elevation, while
vagal stimulation worsens it. (There is a case report of a patient who died while shaving, presumed due to the
vagal stimulation of the carotid sinus massage!) The administration of class Ia, Ic and III drugs increases the
ST segment elevation, and also fever. Exercise decreases ST segment elevation in some patients but
increases it in others (after exercise when the body temperature has risen). The changes in heart rate induced
by atrial pacing are accompanied by changes in the degree of ST segment elevation. When the heart rate
decreases, the ST segment elevation increases and when the heart rate increases the ST segment elevation
decreases. However, the contrary can also be observed.
Characteristics
Characterized by a coved-type ST-segment elevation in the right precordial leads
The Brugada ECG is often concealed, but can be unmasked or modulated by a number of
drugs and pathophysiological states including sodium channel blockers, a febrile state, vagotonic agents,
tricyclic antidepressants, as well as cocaine and Propranolol intoxication.
Genetics
1.
SCN5A is a gene that encodes the alpha sodium unit of the cardiac sodium channel.
Mutations in SCN5A account for about 15-30% of Brugada syndrome cases. A negative genetic test for
SCN5A does not exclude that SCN5A is causing the clinical syndrome because the genetic tests do not
32
evaluate for mutations in promotors, cryptic splicing mutations, or gross rearrangements in the protein
product.
2.
Glycerol-3-phosphate dehydrogenase (GPD1L) is associated with progressive conduction
disease and low sensitivity to procainamide resulting from decreased Isodium current. It has a relatively
good prognosis.
3.
CACNA1C (alpha subunit of L-type cardiac calcium channel) and CACNB2b (beta subunit
of L-type cardiac calcium channel) is associated with a shortened QT interval and a combinatin
Brugada/Short QT interval syndrome.
Brugada EKG
1.
Type 1 ST segment elevation is diagnostic of Brugada syndrome and is characterized by a
coved ST-segment elevation ≥2 mm (0.2 mV) followed by a negative T wave.
2.
Type 2 ST-segment elevation has a saddleback appearance with a high take-off ST-segment
elevation of ≥2 mm followed by a trough displaying ≥1 mm ST elevation followed by either a positive or
biphasic T wave.
3.
Type 3 ST-segment elevation has either a saddleback or coved appearance with an STsegment elevation of <1 mm.
EKG of a Patient with Brugada Syndrome
Lead placements
General characteristics
Brugada Type 1
Brugada Type 1
Brugada
Type 1
Brugada
Type 1
Brugada
Type 1
Brugada
Brugada
Type 2
Brugada Type 1
Type 2
(A) Normal electrocardiogram pattern in the precordial
leads V1-3, (B) changes in Brugada syndrome (type B)
Diagnosis
Diagnosed when a Type 1 ST-segment elevation is observed in more than one right
precordial lead (V1-V3), in the presence or absence of sodium channel blocking agent, and in conjunction
with one or more of the following:
1.
Family history of SCD (<45 years old)
2.
Documented VF
3.
Polymorphic ventricular tachycardia
4.
Coved-type ECGs in family members
33
5.
6.
7.
Inducibility of VT with programmed electrical stimulation (PES)
Syncope
Nocturnal agonal respiration
Diagnosis is also considered positive when a Type 2 (saddleback pattern) or Type 3 STsegment elevation is observed in more than one right precordial lead under baseline conditions and can be
converted to the diagnostic Type 1 pattern occurs upon exposure to sodium channel blocker.
Sodium Challenge
Drugs that can be used
o
Ajmaline 1 mg/kg/5 min IV
o
Flecainide 2 mg/kg/10 min IV or 400 mg PO
o
Procainamide 10 mg/kg/10 min IV
o
Pilsicainide 1 mg/kg/10 min IV
The sodium challenge should be terminated when
1.
Diagnostic Type 1 ST-segment elevation or Brugada ECG, develops
2.
ST segment in Type 2 increases by ≥2 mm
3.
Premature ventricular beats or other arrhythmias develop
4.
QRS widens to ≥130% of baseline
Arrhythmias
1.
Polymorphic VT resembling a rapid Torsade de Pointes (TdP)
2.
Monomorphic VT is observed infrequently
3.
VT/VF often terminates spontaneously in patients with the Brugada syndrome which may
explain why patients wake up at night after episodes of agonal respiration caused by the arrhythmia.
Risk Statification
Patients with syncope and an abnormal Type 1 ECG are at higher risk
Asymptomatic patients at risk can be identified
o
Presence of spontaneous Type 1 ST-segment elevation
o
Characteristics of the S wave
o
Presence of late potentials
o
Inducibility of VT/VF using PES.
Treatment
The cause of death in Brugada syndrome is ventricular fibrillation.The episodes of syncope (fainting)
and sudden death (aborted or not) are caused by fast polymorphic ventricular tachycardias or ventricular
fibrillation. These arrhythmias appear with no warning. While there is no exact treatment modality that
reliably and totally prevents ventricular fibrillation from occurring in this syndrome, treatment lies in
termination of this lethal arrhythmia before it causes death. This is done via implantation of an implantable
cardioverter-defibrillator (ICD), which continuously monitors the heart rhythm and will defibrillate an
individual if ventricular fibrillation is noted. Some recently performed studies had evaluated the role of
quinidine, a Class Ia antiarrythmic drug, for decreasing VF episodes occurring in this syndrome. Quinidine
was found to decrease number of VF episodes and correcting spontaneous ECG changes, possibly via
inhibiting Ito channels. Those with risk factors for coronary artery disease may require an angiogram before
ICD implantation.
Aborted sudden death are at high risk for recurrence and should receive an ICD
VT storm has been successfully treated with Isoproterenol. The mechanism is thought to be
augmenting the cardiac L type channel.
Asymptomatic patients require risk stratification and clinical judegement to help guide
therapy
Quinidine (class IA sodium channel blocker) blocks the Ito current and is proven to suppress
spontaneous VF
Cilostazol (phosphodiesterase III inhibitor that increases inward L type calcium channel
current and reported to suppress spontaneous VF
Bepridil suppress spontaneous VF probably through blocking Ito current
Medical therapy alone with the above agents is currently not evaluated in randomized trials
and should not be used as loan therapy.
Long QT syndrome
The long QT syndrome (LQTS) is a heart condition associated with prolongation of repolarisation
(recovery) following depolarisation (excitation) of the cardiac ventricles. It is associated with syncope
(fainting) and sudden death due to ventricular arrhythmias. Arrhythmias in individuals with LQTS are often
34
associated with exercise or excitement. LQTS is associated with the rare, ventricular arrhythmia torsade de
pointes, which can deteriorate into ventricular fibrillation and ultimately death.
Individuals with LQTS have a prolongation of the QT interval on the ECG. The Q wave on the ECG
corresponds to ventricular depolarization while the T wave corresponds to ventricular repolarization. The QT
interval is measured from the Q point to the end of the T wave. While many individuals with LQTS have
persistent prolongation of the QT interval, some individuals do not always show the QT prolongation; in
these individuals, the QT interval may prolong with the administration of certain medications.
The three most common forms
The QT interval start at the onset of the
A 12 lead ECG of a patient withof LQTS can be recognized by
Q wave and ends where the tangent line
characteristic
ECG
acquired long QT syndrome.the
for the steepest part of the T wave
abnormalities
Notice the QT prolongation. The
intersects with the baseline of the ECG.
QTc is about 640ms.
A bifasic T wave. The tangent to theThe T wave is broad, but the tangent
'hump' with the largest amplitude iscrosses the baseline before the T wave
The ECG does not meet the
chosen. This can change from beatjoins the baseline. The QT interval
baseline after the end of the T
to beat, making it more important towould be overestimated when this last
wave. Still, the crossing of the
average several measurements.
definition of the end of the T wave
tangent and baseline should be
would be used.
used for measurements.
Associated syndromes
A number of syndromes are associated with LQTS.
Jervell and Lange-Nielsen syndrome
The Jervell and Lange-Nielsen syndrome (JLNS) is an autosomal recessive form of LQTS with
associated congenital deafness. It is caused specifically by mutation of the KCNE1 and KCNQ1 genes
In untreated individuals with JLNS, about 50 percent die by the age of 15 years due to ventricular
arrhythmias.
Romano-Ward syndrome
Romano-Ward syndrome is an autosomal dominant form of LQTS that is not associated with
deafness.
Mechanism of arrhythmia generation
All forms of the long QT syndrome involve an abnormal repolarization of the heart. The abnormal
repolarization causes differences in the "refractoriness" of the myocytes. After-depolarizations (which occur
more commonly in LQTS) can be propagated to neighboring cells due to the differences in the refractory
periods, leading to re-entrant ventricular arrhythmias.
It is believed that the so-called early after-depolarizations (EADs) that are seen in LQTS are due to
re-opening of L-type calcium channels during the plateau phase of the cardiac action potential. Since
adrenergic stimulation can increase the activity of these channels, this is an explanation for why the risk of
sudden death in individuals with LQTS is increased during increased adrenergic states (ie exercise,
excitement) -- especially since repolarization is impaired. Normally during adrenergic states, repolarizing
currents will also be enhanced to shorten the action potential. In the absence of this shortening and the
presence of increased L-type calcium current, EADs may arise.
The so-called delayed after-depolarizations (DADs) are thought to be due to an increased Ca2+ filling
of the sarcoplasmic reticulum. This overload may cause spontaneous Ca2+ release during repolarization,
causing the released Ca2+ to exit the cell through the 3Na+/Ca2+-exchanger which results in a net depolarizing
current.
Diagnosis
35
The diagnosis of LQTS is not easy since 2.5% of the healthy population have prolonged QT interval,
and 10% of LQTS patients have a normal QT interval. A commonly used criterion to diagnose LQTS is the
LQTS "diagnostic score". Its based on several criteria giving points to each. With 4 or more points the
probability is high for LQTS, and with 1 or less point the probability is low. Two or 3 points indicates
intermediate probability.
QTc (Defined as QT interval / square root of RR interval)
o
>= 480 msec - 3 points
o
460-470 msec - 2 points
o
450 msec and male gender - 1 point
Torsades de Pointes ventricular tachycardia - 2 points
T wave alternans - 1 point
Notched T wave in at least 3 leads - 1 point
Low heart rate for age (children) - 0.5 points
Syncope (one cannot receive points both for syncope and Torsades de pointes)
o
With stress - 2 points
o
Without stress - 1 point
Congenital deafness - 0.5 points
Family history (the same family member cannot be counted for LQTS and sudden death)
o
Other family members with definite LQTS - 1 point
o
Sudden death in immediate family (members before the age 30) - 0.5 points
Treatment options
There are two treatment options in individuals with LQTS: arrhythmia prevention, and arrhythmia
termination.
Arrhythmia prevention
Arrhythmia suppression involves the use of medications or surgical procedures that attack the
underlying cause of the arrhythmias associated with LQTS. Since the cause of arrhythmias in LQTS is after
depolarizations, and these after depolarizations are increased in states of adrenergic stimulation, steps can be
taken to blunt adrenergic stimulation in these individuals. These include:
Administration of beta receptor blocking agents which decreases the risk of stress induced
arrhythmias. Beta blockers are the first choice in treating Long QT syndrome.
In 2004 it has been shown that genotype and QT interval duration are independent predictors of
recurrence of life-threatening events during beta-blockers therapy. Specifically the presence of QTc >500ms
and LQT2 and LQT3 genotype are associated with the highest incidence of recurrence. In these patients
primary prevention with ICD (Implantable Cardioverster Defibrilator) implantaion can be considered.
Potassium supplementation. If the potassium content in the blood rises, the action potential
shortens and due to this reason it is believed that increasing potassium concentration could minimize the
occurrence of arrhythmias. It should work best in LQT2 since the HERG channel is especially sensible to
potassium concentration, but the use is experimental and not evidence based.
Mexiletine. A sodium channel blocker. In LQT3 the problem is that the sodium channel does
not close properly. Mexiletine closes these channels and is believed to be usable when other therapies fail. It
should be especially effective in LQT3 but there is no evidence based documentation.
Amputation of the cervical sympathetic chain (left stellectomy). This may be used as an addon therapy to beta blockers but modern therapy mostly favors ICD implantation if beta blocker therapy fails.
Arrhythmia termination
Arrhythmia termination involves stopping a life-threatening arrhythmia once it has already occurred.
The only effective form of arrhythmia termination in individuals with LQTS is placement of an implantable
cardioverter-defibrillator (ICD). ICD are commonly used in patients with syncopes despite beta blocker
therapy, and in patients who have experienced a cardiac arrest.
With better knowledge of the genetics underlying the long QT syndrome, more precise treatments
will be readily available.
SADS
SADS, or sudden arrhythmia death syndrome, is a term used to describe sudden death due to
cardiac arrest brought on by an arrhythmia. The most common cause of sudden death in the US is coronary
artery disease. Approximately 300,000 people die suddenly of this cause every year in the US.
Sudden Arrhythmia Death Syndrome (SADS) can also occur from other causes. Also, there are many
inherited conditions and heart diseases that can affect young people that can cause sudden death. Many of
these victims have no symptoms before dying suddenly.
36
Causes of SADS in young people are long QT syndrome, Brugada syndrome, Catecholaminergic
polymorphic ventricular tachycardia and hypertrophic cardiomyopathy and arrhythmogenic right ventricular
dysplasia ("arrythmia"-causing, "right ventricle"-involving, pre-cancerous malformation).
List of common cardiac arrhythmias
Atrial Rhythms
o
Premature Atrial Contractions (PACs)
o
Wandering Atrial Pacemaker
o
Multifocal atrial tachycardia
o
Supraventricular tachycardia (SVT)
o
Atrial flutter
o
Atrial fibrillation (Afib)
Ventricular Rhythms
o
Premature Ventricular Contractions (PVC)
o
Accelerated idioventricular rhythm
o
Ventricular tachycardia
o
Ventricular fibrillation
o
Polymorphic ventricular tachycardia
Atrial Ventricular Arrythmias
o
AV nodal reentrant tachycardia
o
AV reentrant tachycardia
Wolff-Parkinson-White syndrome
Lown-Ganong-Levine syndrome
Junctional Arrhythmias
o
Junctional rhythm
o
Junctional tachycardia
o
Premature junctional complex
Heart Blocks, also known as AV blocks
o
First degree heart block, also known as PR prolongation
o
Second degree heart block
Type 1 Second degree heart block, also known as Mobitz I or Wenckebach
Type 2 Second degree heart block, also known as Mobitz II
o
Third degree heart block, also known as complete heart block
Medical treatment
Initital administration and monitoring of the effect of drugs for treatment of heart rhythm
disorders. Electrophysiologists are often involved when severe or life threatening arrhythmias are being
treated, or when multiple drugs must be used to treat an arrhythmia.
Antiarrhythmic agent
Antiarrhythmic agents are a group of pharmaceuticals that are used to suppress fast rhythms of the
heart (cardiac arrhythmias), such as atrial fibrillation, atrial flutter, ventricular tachycardia, and ventricular
fibrillation.
While the use of antiarrhythmic agents to suppress atrial arrhythmias (atrial fibrillation and atrial
flutter) is still in practice, it is unclear whether suppression of atrial arrhythmias will prolong life.
In the past, it was believed that following myocardial infarction (heart attack), suppression of
ventricular arrhythmias would prolong life. However large clinical trials found that suppression of these
arrhythmias would paradoxically increase mortality, which may happen due to the proarrhythmic effect these
drugs may have (CAST trial).
In individuals with atrial fibrillation, antiarrhythmics are still used to suppress arrhythmias. This is
often done to relieve the symptoms that may be associated with the loss of the atrial component to
ventricular filling (atrial kick) that is due to atrial fibrillation or flutter.
37
Class Ia agent decreasing Vmax,
Effect
of
class
Ib
The cardiac actionthereby increasing action potentialantiarrhythmic agents on the cardiac
duration.
action potential.
potential
Effect of class III antiarrhythmic agent on
Effect of class Ic antiarrhythmic agent on
cardiac
action
potential.
cardiac action potential.
In individuals with ventricular arrhythmias, antiarrhythmic agents are often still in use to suppress
arrhythmias. In this case, the patient may have frequent arrhythmic events or be at high risk for ventricular
arrhythmias. Antiarrhythmic agents may be considered the first-line therapy in the prevention of sudden
death in certain forms of structural heart disease, and failure of these agents to suppress arrhythmias may
lead to implantation of an implantable cardioverter-defibrillator (ICD).
The use of antiarrhythmic agents in this population may be in conjunction with an ICD. In this case,
the ICD is used to prevent sudden death due to ventricular fibrillation, while the antiarrhythmic agent(s) are
used to suppress ventricular tachyarrhythmias so that the ICD doesn't shock the patient frequently.
Many attempts have been made to classify antiarrhythmic agents. The problem arises from the fact
that many of the antiarrhythmic agents have multiple modes of action, making any classification imprecise.
Vaughan Williams antiarrhythmic classification
The Vaughan Williams classification, introduced in 1970, is one of the most widely used
classification schemes for antiarrhythmic agents. This scheme classifies a drug based on the primary
mechanism of its antiarrhythmic effect. However, its dependence on primary mechanism is one of the
limitations of the VW classification, since many antiarrhythmic agents have multiple action mechanisms.
Amiodarone, for example, has effects consistent with all of the first four classes. Another limitation is the
lack of consideration within the VW classification system for the effects of drug metabolites.
Procainamide—a class Ia agent whose metabolite N-acetyl procainamide (NAPA) has a class III action—is
one such example. A historical limitation was that drugs such as digoxin and adenosine – important
antiarrhythmic agents – had no place at all in the VW classification system. This has since been rectified by
the inclusion of class V.
There are five main classes in the Vaughan Williams classification of antiarrhythmic agents:
Class I agents interfere with the sodium (Na+) channel.
Class II agents are anti-sympathetic nervous system agents. All agents in this class are beta
blockers.
Class III agents affect potassium (K+) efflux.
Class IV agents affect the AV node.
Class V agents work by other or unknown mechanisms.
Class I agents
The class I antiarrhythmic agents interfere with the sodium (Na+) channel. Class I agents are grouped
by what effect they have on the Na+ channel, and what effect they have on cardiac action potentials.
Class Ia agents
Class Ia agents block the fast sodium channel.
Blocking this channel depresses the phase 0 depolarization (reduces V max), which prolongs the action
potential duration by slowing conduction. Agents in this class also cause decreased conductivity and
increased refractoriness.
Indications for Class Ia agents are supraventricular tachycardia, ventricular tachycardia, symptomatic
ventricular premature beats, and prevention of ventricular fibrillation.
38
Procainamide can be used in the treatment of atrial fibrillation in the setting of Wolff-ParkinsonWhite syndrome, and in the treatment of wide complex hemodynamically stable tachycardias.
While procainamide and quinidine may be used in the conversion of atrial fibrillation to normal sinus
rhythm, they should only be used in conjunction with an AV node blocking agent (ie: digoxin, verapamil, or
a beta blocker), because procainamide and quinidine can increase the conduction through the AV node and
may cause 1:1 conduction of atrial fibrillation, causing an increase in the ventricular rate.
Class Ia agents include quinidine, procainamide and disopyramide.
Class Ib agents
Class Ib antiarrhythmic agents are sodium channel blockers. Class Ib agents have fast onset and
offset kinetics, meaning that they have little or no effect at slower heart rates, and more effects at faster heart
rates. Class Ib agents shorten the action potential duration and reduce refractoriness. These agents will
decrease Vmax in partially depolarized cells with fast response action potentials. They either do not change
the action potential duration, or they may decrease the action potential duration. Class Ib drugs tend to be
much more specific for voltage gated Na channels than Ia. Lidocane in particular is highly frequency
dependent, in that it has more activity with increasing heart rates. This is because lidocane selectively blocks
Na channels in their open and inactive states and has little binding capability in the resting state.
Class Ib agents are indicated for the treatment of ventricular tachycardia and symptomatic premature
ventricular beats, and prevention of ventricular fibrillation.
Class Ib agents include lidocaine, mexiletine, tocainide, and phenytoin.
Class Ic agents
Class Ic antiarrhythmic agents markedly depress the phase 0 depolarization (decreasing Vmax). They
decrease conductivity, but have a minimal effect on the action potential duration. Of the sodium channel
blocking antiarrhythmic agents (the class I antiarrhythmic agents), the class Ic agents have the most potent
sodium channel blocking effects.
Class Ic agents are indicated for life-threatening ventricular tachycardia or ventricular fibrillation,
and for the treatment of refractory supraventricular tachycardia (ie: atrial fibrillation). These agents are
potentially pro-arrhythmic, especially in settings of structural heart disease (e.g. post-myocardial infarction),
and are contraindicated in such settings.
Class Ic agents include encainide, flecainide, moricizine, and propafenone.
Class II agents
Class II agents are conventional beta blockers. They act by selectively blocking the effects of
catecholamines at the β1-adrenergic receptors, thereby decreasing sympathetic activity on the heart. These
agents are particularly useful in the treatment of supraventricular tachycardias. They decrease conduction
through the AV node.
Class II agents include esmolol, propranolol, and metoprolol.
Class III agents
Class III agents predominantly block the potassium channels, thereby prolonging repolarization.
Since these agents do not affect the sodium channel, conduction velocity is not decreased. The prolongation
of the action potential duration and refractory period, combined with the maintenance of normal conduction
velocity, prevent re-entrant arrhythmias. (The re-entrant rhythm is less likely to interact with tissue that has
become refractory).
Class III antiarrhythmic agents exhibit reverse use dependent prolongation of the action potential
duration (Reverse use-dependence). This means that the refractoriness of the ventricular myocyte increases
at lower heart rates. This increases the susceptibility of the myocardium to early after-depolarizations
(EADs) at low heart rates. Antiarrhythmic agents that exhibit reverse use-dependence are more efficacious at
preventing a tachyarrhythmia than converting someone into normal sinus rhythm. Because of the reverse
use-dependence of class III agents, at low heart rates class III antiarrhythmic agents may paradoxically be
more arrhythmogenic.
Amiodarone is indicated for the treatment of refractory VT or VF, particularly in the setting of acute
ischemia. Amiodarone is also safe to use in individuals with cardiomyopathy and atrial fibrillation, to
maintain normal sinus rhythm. Amiodarone prolongation of the action potential is uniform over a wide range
of heart rates so this drug does not have reverse use-dependent action.7 In contrast, dofetilide blocks only the
rapid K channels; this means that at higher heart rates, when there is increased involvement of the slow K
channels, dofetilide has less of an action potential-prolonging effect.
Sotalol is indicated for the treatment of atrial or ventricular tachyarrhythmias, and AV re-entrant
arrhythmias. Ibutilide is the only antiarrhythmic agent currently approved by the Food and Drug
Administration for acute conversion of atrial fibrillation to sinus rhythm.
39
Class III agents include amiodarone, azimilide, bretylium, clofilium, dofetilide, tedisamil, ibutilide,
sematilide, and sotalol.
Class IV agents
Class IV agents are slow calcium channel blockers. They decrease conduction through the AV node,
and shorten phase two (the plateau) of the cardiac action potential. They thus reduce the contractility of the
heart, so may be inappropriate in heart failure. However, in contrast to beta blockers, they allow the body to
retain adrenergic control of heart rate and contractility.
Class IV agents include verapamil and diltiazem.
Class V agents
Class V agents include digoxin and adenosine. Digoxin increases vagal activity via its central action
on the central nervous system, thus decreasing the conduction of electrical impulses through the AV node
Catheter ablation
Ablation therapy - Catheter based creation of lesions in the heart (with radiofrequency
energy, cryotherapy (destructive freezing), or ultrasound energy) to cure or control arrhythmias (see
radiofrequency ablation). Ablation is usually performed during the same procedure as the electrophysiology
study which induces and confirms the diagnosis of the arrhythmia for which ablation therapy is sought.
"Non-complex" ablations include ablation for arrhythmias such as: AV nodal reentrant
tachycardia, Accessory pathway mediated tachycardia, atrial flutter. These procedures are usually performed
using intracardiac catheters (as are used during an electrophysiologic study), fluoroscopy (a real-time X-ray
camera), and electrical recordings from the inside of the heart.
"Complex" ablations include ablation for arrhythmias such as multifocal atrial tachycardia,
atrial fibrillation, and ventricular tachycardia. In addition to the apparatus used for a "non-complex" ablation,
these procedures often make use of sophisticated computer mapping systems to localize the source of the
abnormal rhythm and to direct delivery of ablation lesions.
Surgical Procedures: Pacemaker and Defibrillator implantation and follow up
Implantation of single and dual chamber pacemakers and defibrillators
Implantation of "biventricular" pacemakers and defibrillators for patients with congestive
heart failure
Implantation of loop recorders (implanted ECG recorders for long term monitoring of ECG
to allow for diagnosis of an arrhythmia)
Clinical follow up and reprogramming of implanted devices.
4.Review Questions:
1.
Etiology and pathogenesis of arrhythmias.
2.
Diagnostics of arrhythmias.
3.
Classification of arrhythmias.
4.
Treatment of arrhythmias.
5.
Etiology and pathogenesis of blockades.
6.
Diagnostics of blockades.
7.
Classification of blockades.
8.
Treatment of blockades.
5 Practical tasks:
Work № 1. ECG
Work № 2. Clinical tasks.
40
6. Literature:
1. Braunwald Eugene, P. Zipes Douglas P., Libby Peter, Bonow Robert. Heart Disease: A
Textbook of Cardiovascular Medicine, Single Volume. 7th ed. Saunders W. B., 2004. 2183
p.
2. Taylor R. Cardiovascular Diseases: A Handbook. Springer, 2005. 294 p.
3. Topol Eric J, Califf Robert M, Prystowsky Eric N, Thomas James D, Thompson Paul D.
Textbook of Cardiovascular Medicine. 3rd ed. Lippincott Williams & Wilkins, 2006. 1664 p.
4. Morris Francis, Edhouse June, Brady William J, Camm John. ABC of Clinical
Electrocardiography. BMJ Publishing Group, 2003. 80 p.
5. Hosenpud Jeffrey D. Congestive Heart Failure
Third Edition. Lippincott Williams & Wilkins, 2007. 833 p.
6. Jones Shirley A. ECG Notes: Interpretation And Management Guide. F. A. Davis Company,
2005. 210 p.
7. Feigenbaum Harvey, Armstrong William F., Ryan, Thomas. Echocardiography, 6th Edition.
Lippincott Williams & Wilkins, 2005. 773 p.
8. Fuster Valentin, Alexander R. Wayne, O’Rourke Robert A., Roberts Robert, King Spencer
B., Prystowsky Eric N., Nash Ira. Hurst’s The Heart, 11th Edition. McGraw-Hill
Professional, 2004. 2400 p.
9. Hampton John R. The ECG in Practice. Churchill Livingstone, 2003. 325 p.
10. Kasper Dennis L., Braunwald Eugene, Fauci Anthony, Hauser Stephen, Longo Dan,
Jameson J. Larry. Harrison’s Principles of Internal Medicine, 16th ed. McGraw-Hill
Professional, 2004. 2800 p.
11. Boon Nicholas A., Colledge Nicki R., Walker Brian R., Hunter John. Davidson’s Principles
and Practice of Medicine. Churchill Livingstone, 2006. 1392 p.
41