HW- Inorganic chemis..
advertisement
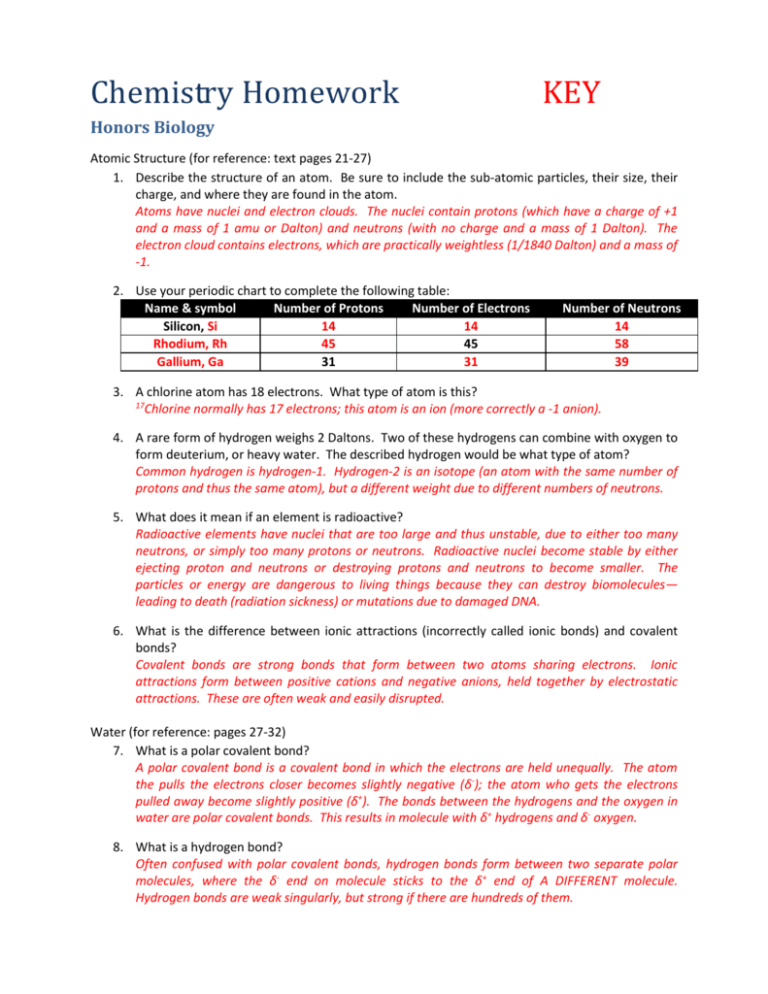
Chemistry Homework KEY Honors Biology Atomic Structure (for reference: text pages 21-27) 1. Describe the structure of an atom. Be sure to include the sub-atomic particles, their size, their charge, and where they are found in the atom. Atoms have nuclei and electron clouds. The nuclei contain protons (which have a charge of +1 and a mass of 1 amu or Dalton) and neutrons (with no charge and a mass of 1 Dalton). The electron cloud contains electrons, which are practically weightless (1/1840 Dalton) and a mass of -1. 2. Use your periodic chart to complete the following table: Name & symbol Number of Protons Number of Electrons Silicon, Si 14 14 Rhodium, Rh 45 45 Gallium, Ga 31 31 Number of Neutrons 14 58 39 3. A chlorine atom has 18 electrons. What type of atom is this? 17 Chlorine normally has 17 electrons; this atom is an ion (more correctly a -1 anion). 4. A rare form of hydrogen weighs 2 Daltons. Two of these hydrogens can combine with oxygen to form deuterium, or heavy water. The described hydrogen would be what type of atom? Common hydrogen is hydrogen-1. Hydrogen-2 is an isotope (an atom with the same number of protons and thus the same atom), but a different weight due to different numbers of neutrons. 5. What does it mean if an element is radioactive? Radioactive elements have nuclei that are too large and thus unstable, due to either too many neutrons, or simply too many protons or neutrons. Radioactive nuclei become stable by either ejecting proton and neutrons or destroying protons and neutrons to become smaller. The particles or energy are dangerous to living things because they can destroy biomolecules— leading to death (radiation sickness) or mutations due to damaged DNA. 6. What is the difference between ionic attractions (incorrectly called ionic bonds) and covalent bonds? Covalent bonds are strong bonds that form between two atoms sharing electrons. Ionic attractions form between positive cations and negative anions, held together by electrostatic attractions. These are often weak and easily disrupted. Water (for reference: pages 27-32) 7. What is a polar covalent bond? A polar covalent bond is a covalent bond in which the electrons are held unequally. The atom the pulls the electrons closer becomes slightly negative (δ-); the atom who gets the electrons pulled away become slightly positive (δ+). The bonds between the hydrogens and the oxygen in water are polar covalent bonds. This results in molecule with δ+ hydrogens and δ- oxygen. 8. What is a hydrogen bond? Often confused with polar covalent bonds, hydrogen bonds form between two separate polar molecules, where the δ- end on molecule sticks to the δ+ end of A DIFFERENT molecule. Hydrogen bonds are weak singularly, but strong if there are hundreds of them. 9. Define cohesion and adhesion. Why is the cohesiveness of water important to living things? Cohesion is water’s ability to hydrogen bond and stick to other water molecules. Adhesion is water’s ability to hydrogen and stick to other molecules. Water’s cohesiveness leads to the phenomena of capillary action (important to the circulatory system in plants) and surface tension, very important for water-strider insects or emerald basilisks. 10. Define pH. Include a sketch of the pH scale, and show where you would find acidic, basic, and neutral compounds. pH is a measure of the amount of hydrogen ions, H+, in a solution. Chemicals with high levels of H+ and low levels of a companion ion, OH-, are acids. Chemicals with equal numbers of H+ and OH- are neutral. Chemicals with more OH- than H+ are bases. The pH scale is line centered at 7, which is neutral (like pure water). Acids have a pH of less than 7, and bases a pH greater than 7. 11. The pH scale is logarithmic. What is the difference in H+ concentration between an acid with a pH of 1 and an acid with a pH of 3? Each step of the pH scale is a power of 10; a pH 1 is 102 or 100 times more powerful than pH 3. 12. What is unique about solid water compared to liquid water? Why is this important to biology? Solid water is less dense then liquid water, so ice floats. This insulates the water under the ice, preventing it from freezing solid and killing everything living in it. 13. What is special about the heat capacity of water? The high heat capacity of water means that it heats up and cools down very slowly. This keeps the temperature of the oceans, lakes, and ponds fairly stable, and keeps the life living in them from experiencing too much thermal stress. Review 14. Draw the electron shell arrangement of Silicon (Si), Phosphorus (P), and Magnesium (Mg). Si 2e- 8e- 4eP 2e- 8e- 5eMg 2e- 8e- 2e15. What type (anion or cation) and magnitude of ion would you expect Beryllium (Be), Sulfur (S), and Potassium (K) to make? Beryllium makes a +2 cation; Sulfur a -2 anion, and Potassium a +1 cation. 16. Nitrogen forms a covalent bond with hydrogen. How many hydrogens are needed for nitrogen to form a stable molecule? Nitrogen has five valence electrons; it needs three more. It would covalently bond to share electrons with three hydrogens to complete its valence shell 17. Explain the difference between a polar covalent bond and a hydrogen bond. A polar covalent bond is found inside one molecule. It forms when two atoms in a bond share electrons unequally, resulting in one end of the molecule have a slightly positive (δ+) charge and the other end a slightly negative (δ-) charge. Hydrogen bonds form between two molecules with polar covalent bonds. They are the weak attraction between the δ+ end of one molecule and the δ- end of another molecule.









