Homework 2
advertisement

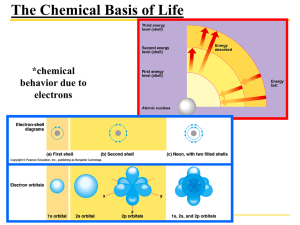
Homework Assignment #2 BIO105K NAME _____________________ 1) _____ Since the electronegativity of oxygen and hydrogen atoms are different, the covalent bonds in a water molecule are: A. ionic B. polar C. hydrophobic D. hydrogen bonds E. isotopes 2) _____ Which of the following pH values represents the greatest concentration of H+ ions? A. 4 B. 7 C. 2 D. 12 E. 10 3) _____ What are the four essential elements that make up 96% of living matter? A. Bromine, Calcium, Zinc, Iron B. Oxygen, Carbon, Hydrogen, Nitrogen C. Iron, Calcium, Carbon, Potassium D. Carbon, Hydrogen, Sodium, Potassium E. Magnesium, Chlorine, Sodium, Carbon 4) _____ The more electronegative an atom is: A. the stronger it pulls toward shared electrons B. the larger its atomic mass C. the less radioactive it is D. the easier it loses an electron E. the more neutrons it has 5) _____ Which of the following affect radioactivity? A. electrons B. neutrons C. ions D. protons E. atomic number 6) ____ How many protons are in the atom of sodium shown above? A. 1 B. 10 C. 11 D. 12 E. 23 7) ____ How many electrons are in the atom of sodium shown above? A. 1 B. 10 C. 11 D. 12 E. 23 8) _____ Why does ice float in liquid water? A. because there are no hydrogen bonds in ice B. because ice is less dense than water C. because ice is a solid D. because ice is pushed up by water E. because the covalent bonds are broken in ice 9) _____ Hydrophilic substance are________ and hydrophobic substances are __________. A. Water loving; water fearing. B. Polar; non-polar C. Soluble in water; soluble in lipid D. A, B, and C are all true E. none of the above 10) _____ How many grams of glucose would be in 2 liters of a 1 M solution ofglucose? (the molecular weight of glucose is 180) A. 2 B. 90 C 180 D 360 E. need more information