Answers of Self Assessment Test
advertisement
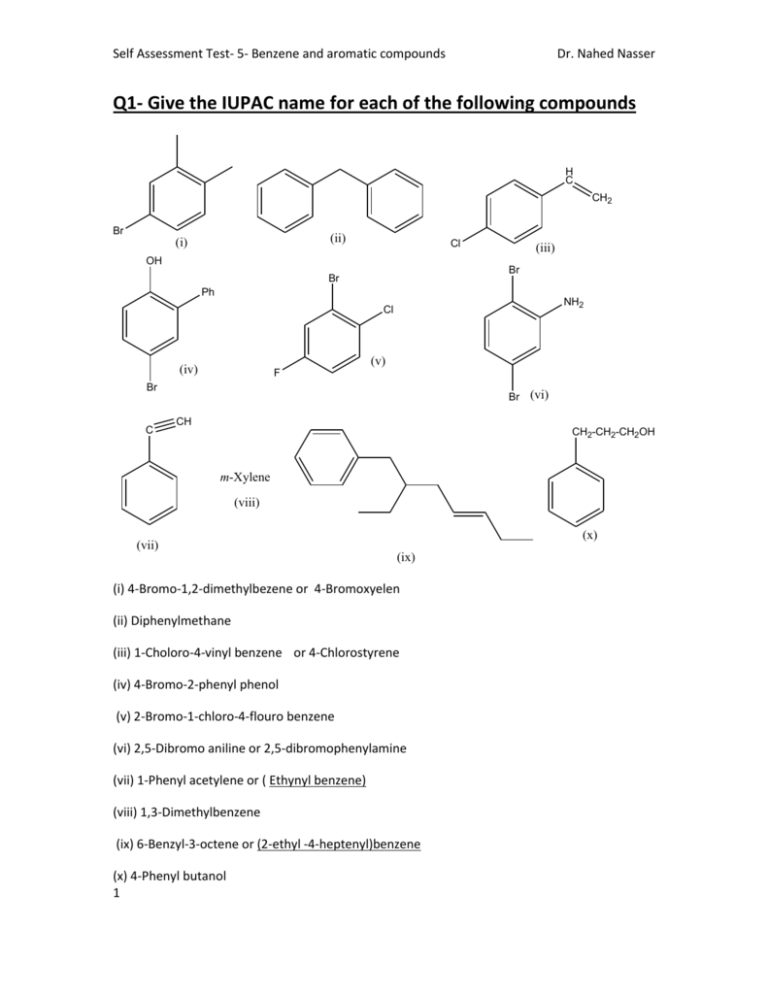
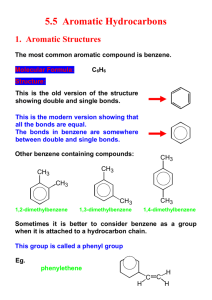
Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Q1- Give the IUPAC name for each of the following compounds H C CH2 Br (ii) (i) Cl OH (iii) Br Br Ph NH2 Cl (v) (iv) F Br C Br (vi) CH CH2-CH2-CH2OH m-Xylene (viii) (x) (vii) (ix) (i) 4-Bromo-1,2-dimethylbezene or 4-Bromoxyelen (ii) Diphenylmethane (iii) 1-Choloro-4-vinyl benzene or 4-Chlorostyrene (iv) 4-Bromo-2-phenyl phenol (v) 2-Bromo-1-chloro-4-flouro benzene (vi) 2,5-Dibromo aniline or 2,5-dibromophenylamine (vii) 1-Phenyl acetylene or ( Ethynyl benzene) (viii) 1,3-Dimethylbenzene (ix) 6-Benzyl-3-octene or (2-ethyl -4-heptenyl)benzene (x) 4-Phenyl butanol 1 Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Q2- Draw the structure of the following compounds CH3 NH2 OH O2N NO2 NO2 m-Diisobutylbenzene 2,4,6-Trinitrotoluene NH2 p-Aminophenol p- Ethylaniline Br Cl Benzyl bromide o-Chlorostyrene Br m-Bromoisopropylbenzene OH Br Cl 4-Bromo-2-chloro-1-ethylbenzene 4-Benzylhexyne o-Xylene CN 4-Allyl-3-cyanophenol 2 Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Q3. Provide the product (s) of the following chemical reactions: (CH3)2CHCl O2N O2N AlCl3 O O Br2 / FeBr3 Br O O Cl + AlCl3 O NO2 NH2 NH2 HNO3 / H2SO4 NH2 + O2N COOH KMnO4 / Heat COOH OC2H5 SO3 / H2SO4 + SO3H 3 OC2H5 OC2H5 HO3S Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Br Br2 / UV light (Major product) O2N CH3 KMnO4 / Heat Br i)Mg / dry ether ii) H2O / H 4 O2N COOH Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Q4. Indicate whether the following structure is aromatic or not aromatic? Give reason in each case. CH3 Not aromatic it has a sp3 carbon atom Not aromatic because: n=1.5 Aromatic because: Not aromatic cyclic, planar, each c-atomithas haspaorbital sp3 and n=1 carbon atom Br Not aromatic because: n=1.5 Aromatic because: cyclic, planar, each c-atom has p orbital and n=1 Not aromatic it has a sp3 carbon atom Not aromatic it has two sp3 carbon atoms Not aromatic because: n=2.5 Aromatic because: cyclic, planar, each c-atom has p orbital and n=2 5 Aromatic because: In each ring cyclic, planar, each catom has p orbital and n=1 Aromatic because: cyclic, planar, each c-atom has p orbital and n=1 Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser Q5- Show how would you obtain the following compounds via electrophilic aromatic substitution reaction(s) HNO4 / H2SO4 O2N COOH COOH SO3 / H2SO4 CH3 Br Cl HO3S CH3 CH3 Br / AlCl3 O CH3 CH3 O CH3Cl / AlCl3 NO2 NO2 (CH3)2CHCH2CH2Cl / AlCl3 CH3Cl / AlCl3 H3CO HOOC H3CO CH3 HOOC CH3Cl / AlCl3 Cl O CH3CH2CH2CH2COCl / AlCl3 O PhCOCl / AlCl3 6 Self Assessment Test- 5- Benzene and aromatic compounds Dr. Nahed Nasser 6-Multiple choice questions: (a) The electrophile in the acylation of toluene is i) CH3Cl ii) CH3 iii) CH3CO iv) CH3COCl (b) All bonds in benzene are i) Polarized ii) Localized iii) Delocalized iv) Ionic (c) Length of bonds in benzene ring equals i) 1.54 Ǻ ii) 1.39 Ǻ iii) 1.34 Ǻ iv) 1.50 Ǻ (d) Benzene is more stabilized than 1,3,5-cyclohexatriene by i) 36 Kcal/mol 7 ii) 85.8 kcal/mol iii) 55.2 kcal/mol iv) 28.6 kcal/mol