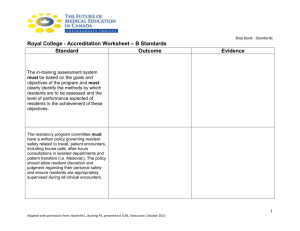
Residency Training Manual in General Pathology
advertisement

RESIDENCY TRAINING PROGRAM IN GENERAL PATHOLOGY DEPARTMENT OF LABORATORY MEDICINE AND PATHOLOGY UNIVERSITY OF ALBERTA Program Director: L. Puttagunta, MD Associate Professor of Pathology Revised September 2008 1 INTRODUCTION The training manual is designed to provide residents in the General Pathology Residency Training Program with an outline of the general and specific objectives of the program and of the rotations available. The manual is also intended to provide an overview of the facilities available to you. The manual serves secondarily as a guide for faculty teaching in the program. The training program is structured to ensure that residents obtain the experience necessary to fulfil the requirements of the Royal College for certification. However, we also attempt to provide for the special interests or needs of individual residents. The program is based at a number of institutions which include the University of Alberta Hospital, DynaLIFE DX Diagnostic Laboratory Services, the Royal Alexandra Hospital, the Misericordia Community Hospital, the Grey Nuns Hospital, the Cross Cancer Institute and the Medical Examiner’s Office. There are program coordinators at all of these institutions. The program has both didactic and apprenticeship components and residents are expected to make full use of both. Lists of available didactic teaching sessions, rounds and seminars are included in the manual and you are encouraged to attend, where possible. There are ample library facilities within the Department and in the hospital and university. However, please remember that, while the program provides the learning opportunities, experience, and facilities, the resident remains responsible for utilizing these to their fullest. Enclosed are the Royal College requirements for training and our own objectives for the program. Evaluation will be based on these objectives and on your performance on rotations; you will be provided with regular feedback on your progress. The teaching faculty hopes that you enjoy and profit from your training and the program welcomes your feedback at all times. You are entering an exciting new phase of your career, and we are eager to help you in every way possible in your training. Director General Pathology Training Program 2 ORGANIZATION OF PROGRAM The training program is led by the Program Director with the assistance of the Program Administrator and the General Pathology Residency Program Committee. The General Pathology Program Committee meets regularly (four times during the academic year) and major problems may be brought to the committee’s attention by contacting the resident representatives, or any of its members. GENERAL PATHOLOGY RESIDENCY PROGRAM COMMITTEE TERMS OF REFERENCE The Residency Program Committee (RPC) in General Pathology is responsible to the Chair of the academic Department of Laboratory Medicine and Pathology and to the Associate Dean responsible for Postgraduate Medical Education. The RPC is responsible for policy and operation of the Residency Training Program. The Committee is made up of the Program Director as Chair, faculty representing the principal areas of training and practice in General Pathology, and the Program Administrator. At least one faculty member from each participating institution will sit on the Committee. A resident representative sits on this committee; however, all residents are welcome to attend the regular Committee meetings. It is the function of the Committee: 1. to recommend policies regarding all aspects of education for the training program, 2. to formulate the procedures whereby the Royal College of Physicians and Surgeons of Canada, Faculty and Departmental policies are met, 3. to survey the overall quality of the Residency Program by regular review and to make recommendations as may be appropriate, 4. to maintain liaison and communication with the Royal College of Physicians and Surgeons of Canada regarding the organization and conduct of the Residency Training Program, 5. to maintain liaison and communication with the Director of Postgraduate Medical Education and the Postgraduate Medical Education Committee of the Faculty of Medicine. 6. to maintain liaison and communication with other residency programs in Canada, 7. to ensure that there is a regular evaluation of resident performance according to Departmental and Faculty policy and to review, twice annually, the progress of each resident in the program, 8. to make recommendations to the Program Director for acceptance of candidates into the program . 3 RESIDENCY PROGRAM COMMITTEE IN GENERAL PATHOLOGY 2008 - 2009 Dr. Fiona Bamforth (UAH) Dr. Stuart Brown (DynaLIFE) Dr. Greg Charrois (Chief Resident) Dr. Gwen Clarke (UAH) Dr. John Danyluk (MCH) Dr. Kinga Kowalewska (UAH) Dr. Carolyn O’Hara (DynaLIFE) Dr. Lakshmi Puttagunta (Chair) (UAH) Mrs. Susan Baert (UAH) Ex Officio: Associate Dean for Postgraduate Medical Education. 4 SELECTION OF RESIDENTS The program only recruits residents through a national match organized by the Canadian Resident Matching Service (CaRMS) or through the Alberta International Medical Graduate Program. The first iteration of the CaRMS match is restricted to Canadian citizens or landed immigrants. Applicants must be graduates of medical schools accredited by the Liaison Committee on Medical Education (LCME) and must have no previous post-MD clinical training. Residents are selected/ranked on the basis of academic qualifications, previous performances, letters of reference and on-site interviews. 5 RCPSC Specific Standards of Accreditation for Residency Programs in General Pathology Adopted by Council in April 1998 INTRODUCTION The purpose of this document is to provide program directors and surveyors with an interpretation of the general standards of accreditation as they relate to the accreditation of programs in general pathology. This document should be read in conjunction with the booklet General Standards of Accreditation and the document Objectives of Training and Specialty Training Requirements in General Pathology. STANDARD I: ADMINISTRATIVE STRUCTURE There must be an appropriate administrative structure for each residency program. Please refer to Standard B.I in the booklet General Standards of Accreditation for the interpretation of this standard. STANDARD II: GOALS AND OBJECTIVES There must be a clearly worded statement outlining the goals of the residency program and the educational objectives of the residents. The general goals and objectives for general pathology are outlined in the document Objectives of Training and Specialty Training Requirements in General Pathology. Based upon these general objectives each program is expected to develop rotation specific objectives suitable for that particular program, as noted in Standard B.II of the booklet General Standards of Accreditation. Contents STANDARD III: CONTENT AND ORGANIZATION OF THE RESIDENCY PROGRAM There must be an organized program of rotations and other educational experiences, both mandatory and elective, designed to provide each resident with the opportunity to fulfill the educational requirements and achieve competence in the specialty. The content and organization of each accredited program in general pathology must be consistent with the specialty training requirements as outlined in the document Objectives of Training and Specialty Training Requirements in General Pathology. In addition to offering the components noted in the specialty training requirements all accredited programs in general pathology must offer community-based learning experiences. The resident must be provided with a graduated increase in individual professional responsibility, under appropriate supervision, appropriate to the level of competence and experience. 6 STANDARD IV: RESOURCES There must be sufficient resources including teaching faculty, the number and variety of patients, physical and technical resources, as well as the supporting facilities and services necessary to provide the opportunity for all residents in the program to achieve the educational objectives and receive full training as defined by the specialty training requirements in general pathology. In those cases where a university has sufficient resources to provide most of the training in general pathology but lacks one or more essential elements, the program may still be accredited provided that formal arrangements have been made to send residents to another accredited residency program for periods of appropriate prescribed training. Learning environments must include experiences that facilitate the acquisition of knowledge, skills, and attitudes relating to aspects of age, gender, culture, and ethnicity appropriate to general pathology. 1. Teaching Faculty There must be a sufficient number of qualified teaching staff to supervise residents and provide teaching in the basic and clinical sciences related to laboratory medicine. There should be ongoing exposure to general pathologists, initiated at the commencement of training, who can serve as role models during the formative years of the general pathologist's training. Professional staffing (pathologists, pathology assistants, technologists and other personnel), should be sufficient that service work and academic endeavors and roles of the department can be achieved whether or not residents are present in the department. 2. Volume and Variety of Pathological Material While there are no specified minimum numbers of autopsies, surgical/cytology specimens, or forensic work, the volume and diversity of work available for teaching must be adequate to attain the educational objectives of the program. Material provided in the clinical disciplines must also be of sufficient variety to fulfill the stated training objectives. The program must provide an opportunity for the resident to acquire a level of competence in the practice of laboratory medicine appropriate to direct laboratories in community hospital or free-standing laboratories with the capability of recognizing those instances where material and/or cases should be referred. 3. Laboratory Components of the Program The laboratory program must provide adequate resources to ensure satisfaction of the training objectives in individual domains encompassed by 7 laboratory medicine. It is important for the general pathology resident to understand the statistical and analytical bases of laboratory testing, test interpretation, and effective laboratory. Training in general pathology must include: a. Anatomic Pathology i. Autopsy Pathology There must be adequate numbers of autopsies available to provide full training in gross autopsy techniques, histotechniques, photographic, and postmortem microbiological techniques. Instruction in postmortem prosection must be provided under the direction of staff pathologists. Departments of Pathology must ensure prompt resident interpretation and reporting of autopsy findings including clinicopathological correlation. Completed autopsy protocols should be in the patient's medical record within three months after the date of autopsy. ii. Surgical Pathology There must be an adequate volume and range of surgically excised tissues to provide training in the gross examination, dissection, and selection of appropriate tissue blocks for histological study. Experience in biopsy and frozen section interpretation is essential. Experience in the range of histological diagnoses within the range of tissues sent for study in community hospital or free-standing laboratories is essential. iii. Cytopathology There must be an adequate volume and mix of cytologic specimens and facilities available for training in cytology, including aspiration cytology and exfoliative cytology. iv. Forensic Pathology Residents must obtain experience in the special procedures which may be associated with medicolegal autopsies. If necessary to provide such experience, rotations or other arrangements must be available to residents at an appropriate forensic centre or laboratory. A well structured program, one month at least, must be implemented. v. Technical and Special Methods In addition to adequate facilities and equipment for routine fixation and staining of tissues, there must be adequate 8 opportunity to develop knowledge of the applications of special staining procedures. Competence in the operation of the light microscope must be assured, including the applications of polarizing optics and fluorescent microscopy. Residents must acquire a basic knowledge of the principles and techniques of morphometry, molecular pathology, and flow cytometry, and their interpretation. b. Hematopathology There must be adequate experience in the following areas of hematopathology: morphology, immunohematology, blood banking, hemostasis and anticoagulation. Emphasis should be placed on the interpretation of laboratory results. c. Medical Biochemistry There must be adequate experience in the following areas of medical biochemistry: test ordering and patient preparation, methodology, quality control, interpretation of biochemical findings. d. Medical Microbiology There must be adequate experience in the following areas of medical microbiology: bacteriology, antimicrobial sensitivity testing, parasitology, mycology, virology, and infection control. e. Additional areas common to more than one of the above domains include the following: i. Immunology and immunopathology. ii. Medical genetics and principles of cytogenetic analysis. iii. Pediatric pathology which must be supported by an adequate volume and variety of teaching material and appropriate faculty. iv. Laboratory administration; instruction and experience in managing of laboratories of secondary care facilities must be available. v. Information management and data processing applicable to laboratories. 4. Consultation The resident must have ongoing opportunity to act as a consultant to clinical colleagues on the interpretation and clinical relevance of laboratory findings. 5. Community Learning Experiences Community experiences must be available which provide a learning environment with appropriate supervision based on rotation specific objectives. This assumes administrative support and linkages with the 9 program. Exposure to laboratories in smaller community hospitals and free-standing laboratories should be an integral part of the program. The program must include training in facilities with laboratory physicians practising general pathology. 6. Supporting Services — Clinical, Diagnostic, Technical Accredited programs in other disciplines of laboratory medicine are beneficial but not essential for an adequate general pathology program. The integration of general pathology residency with other active teaching services in general surgery, internal medicine, pediatrics, obstetrics and gynecology and psychiatry, through the attendance at rounds and interaction with residents and staff in these other disciplines is to be encouraged. Integration of the general pathology program with the family medicine program is also advantageous. STANDARD V: ACADEMIC AND SCHOLARLY ASPECTS OF THE PROGRAM The academic and scholarly aspects of the program must be commensurate with the concept of a university postgraduate education. The quality of scholarship in the program will in part, be demonstrated by a spirit of enquiry during clinical discussions, rounds, and conferences. Scholarship implies an in-depth understanding of basic mechanisms of normal and abnormal states and the application of current knowledge to practice. 1. Organized Scholarly Activities Organized scholarly activities such as journal clubs, research conferences and seminars must be a regular part of the program. Provision of rounds in each of the specialty areas on a regular basis should be an integral part of the program and residents should be encouraged to attend clinical conferences provided by clinical departments, and if possible, participate in presenting the laboratory component. 2. Basic and Clinical Sciences Relevant to General Pathology The academic program must include organized teaching in the basic and clinical sciences relevant to the specialty. 3. Biomedical Ethics The academic program must ensure that residents gain an understanding of the basic principles and practice of biomedical ethics as it relates to general pathology. 4. Communication Skills The program must ensure that residents learn effective communication skills 10 for interacting with colleagues, co-workers from other disciplines and students. Clearly defined educational objectives for teaching these skills and mechanisms of formal assessment should be in place. 5. Patient Care Team Residents must be given opportunities to develop effective skills in collaborating with all members of the patient care team. 6. Teaching Skills Residents must be given opportunities to develop effective teaching skills by teaching junior colleagues and students, as well as through conference presentations, clinical and scientific reports, and patient education. 7. Management Skills Residents must be given opportunities to develop skills in management as applied to general pathology. Residents should also be prepared for their role as a health care advocate. 8. Quality Assurance/Improvement The program must provide residents with opportunities to gain an understanding of the principles and practice of quality assurance monitoring as it applies to the appropriate utilization of the laboratory and specifically quality control programs applicable to each area of the laboratory. General pathology residents must participate in quality assurance programs in both the laboratory and in the hospital setting. 9. Research Opportunities for Residents There must be a faculty member with the responsibility to facilitate the involvement of residents in research and other scholarly work. The academic program must provide the opportunity for residents to learn biostatistics and the critical appraisal of research methodology and medical literature. Such teaching must include issues related to age, gender, culture, and ethnicity in research protocols and data presentation and discussion. The resident should be encouraged to participate in research programs in the departments to which he or she is assigned. Participation in a research program with the publication of papers or presentation or research findings at medical meetings is to be encouraged. 10. Faculty Research A satisfactory level of research and scholarly activity must be maintained among the faculty identified with the program. 11. Life-Long Learning 11 All programs must promote development of skills in self-assessment and selfdirected life-long learning. To promote this end, the program should provide opportunities for residents to attend conferences outside their own university. STANDARD VI: EVALUATION OF RESIDENT PERFORMANCE There must be mechanisms in place to ensure the systematic collection and interpretation of evaluation data on each resident enrolled in the program. Please refer to Standard B.VI in the booklet General Standards of Accreditation for the interpretation of this standard. 12 RCPSC Objectives of Training and Specialty Training Requirements in General Pathology Approved by Education Committee, 2000 (Please also see Policies and Procedures for Certification and Fellowship) Contents Objectives of Training Definition General objectives Specific objectives Specialty training requirements Objectives of Training DEFINITION General Pathology is that branch of medicine concerned with all aspects of laboratory investigation in health and disease. The discipline incorporates both morphological and non-morphological diagnostic techniques in the areas of Anatomic Pathology, Medical Biochemistry, Medical Microbiology, Hematopathology, and Transfusion Medicine. Contents GENERAL OBJECTIVES On completion of the education program, the residents will be competent to function as consultants in General Pathology and medical laboratory directors. This will require acquisition of a sufficient level of skill in the separate disciplines of Anatomic Pathology, Medical Biochemistry, Medical Microbiology, Hematopathology and Transfusion Medicine to serve as a consultant within the context of a regional or community hospital. Contents SPECIFIC OBJECTIVES (Revised into CanMEDS format — May 2000) At the completion of training, the residents will have acquired the following competencies and will function effectively as: Medical expert General Requirements o o o Demonstrate diagnostic and therapeutic skills for ethical and effective patient care. Access and apply relevant information to clinical practice. Demonstrate effective consultation services with respect to patient care, education and legal opinions. At the completion of training, the residents will demonstrate the diagnostic skills required for ethical and effective patient care and will demonstrate effective consultation skills with respect to patient care, education and legal opinions. The level of knowledge in all areas will reflect the needs of 13 community or regional laboratories. It is not expected that the residents will have the same depth or breadth of knowledge in the laboratory medicine specialties as residents trained in a single discipline. From a functional standpoint the General Pathology residents must be able to recognize and diagnose common morphological and clinical entities. "Common" may be defined as likely to occur one or more times in the course of a year in a laboratory medicine population base of 50,000. The residents must appreciate those clinical situations, cases or specimen types which will require referral to a laboratory medicine specialist working in a tertiary hospital setting. Specific Requirements D. Anatomic Pathology While the general pathology residents must be familiar with and able to characterize disease processes in a wide range of tissues they must develop particular skills in diagnosing common entities in the realms of dermatopathology, gynecological pathology, breast pathology, male genitourinary pathology, lower urinary tract pathology, gastrointestinal (including hepatobiliary and pancreatic) pathology, thyroid and parathyroid pathology, respiratory tract pathology, autopsy pathology, morphologic hematology (peripheral blood, lymph nodes, bone marrow biopsies and spleen), and cytopathology (both fine needle and exfoliative). The residents will recognize the type of case which, because of tissue type, rarity, complexity, or therapeutic implications will require referral to a tertiary centre. The residents will also appreciate those cases where consultation with General Pathology colleagues would be advisable. The expected knowledge base will include: i. ii. iii. iv. v. vi. vii. viii. Normal anatomy and its common variants with a basic understanding of embryological development. The normal gross and light microscopic appearance of tissues both as intact organs and biopsy material. The principles of tissue fixation and preparation of specimens for microscopic examination. The normal appearance of tissue cells in common fixatives, exfoliated or obtained by needle aspiration. The principles of cell biology, immunology, medical genetics and pathogenic mechanisms with an understanding of changes seen in disease states. The principles of light microscopy including polarization, dark field and fluorescence microscopy. The principles of specialized histology techniques including histochemical, immunocytochemical, flow cytometry, morphometry, and hybridization techniques and their application in diagnosis. The rules regarding retention of specimens and processed surgical material as well as the retention of records. 14 ix. x. xi. xii. xiii. xiv. The rules governing consent for postmortem examination and the types of cases which must be reported to the coroner or medical examiner. The definitions of cause, mechanism and manner of death. The sampling of tissues and fluids for the toxicological examination and the legal requirements for the handling of these samples. The recognized standards of workplace safety and the rules governing transportation of dangerous goods. The utilization of ancillary techniques such as biochemical, microbiological, photographic, and radiological studies in surgical and forensic pathology. The principles of quality assurance pertinent to surgical and autopsy pathology. The residents will develop and be able to demonstrate the following skills by the conclusion of the residency program: xv. To recognize and accurately diagnose a broad range of common inflammatory and neoplastic conditions on both histological and cytological material. xvi. To provide appropriate strategies for biopsy (histological and cytological), tissue handling, and reporting to include the features of prognostic and therapeutic importance. xvii. To describe appropriate handling, dissection, and sampling of those tissues normally received for examination by regional or community hospital laboratories. xviii. To be capable of offering a competent intraoperative consultation (frozen section/ imprint/ cytological) with an understanding of the appropriate use and limitations of these procedures. xix. To obtain satisfactory photomicrographs and photographs of gross specimens. xx. To perform a complete postmortem examination with appropriate descriptions at the gross and microscopic levels and incorporating all clinical information. xxi. To be capable of undertaking a complete forensic autopsy in all common situations excluding homicide. This will require knowledge of relevant autopsy techniques and expected findings as well as of the practical aspects of establishing time of death and identifying remains. E. Medical Biochemistry Medical Biochemistry is the study and measurement of biochemical abnormalities in human disease and forms a core component of the training program in general pathology. In all specialties, but especially those that are technologically driven, there is a rapid evolution to be expected in diagnostic test methodology. In addition, as understanding of biochemical 15 abnormalities increases, there is a constant need to update and expand test menus in order to provide rational and efficient strategies to confirm or exclude disease. The increasing availability of new technologies is also expected to blur the distinction between traditional clinical disciplines, enhance the capabilities of community or regional hospital laboratories, and increase the consultative role of the General Pathologist. The discipline of Medical Biochemistry as it pertains to General Pathology involves the following major areas of activity: The supervision and direction of the clinical biochemistry laboratory at the level of a community or regional hospital. The provision of consultation services to clinical colleagues with respect to appropriate and effective biochemical testing strategies and their interpretation. At completion of training, the residents will have a broad knowledge of biochemical testing and laboratory instrumentation pertinent to supervising a community or regional hospital laboratory and offering consultative services to clinical colleagues. The residents must understand the common disorders of, and test strategies pertinent to diagnosis of: Body water and electrolytes Acid-base control Renal function Liver function Lipid disorders Bone disease Pancreatic function and digestive disease Cardiac disease and hypertension Blood sugar control Iron, porphyrin and bilirubin metabolism Endocrine function (especially thyroid, parathyroid, gonadal, pituitary, adrenal) Uric acid metabolism Protein metabolism Common genetic disorders of metabolism A more basic knowledge of pediatric and prenatal clinical biochemistry, nutrition, cancer-associated biochemical abnormalities, therapeutic drug monitoring, pharmacokinetics, and toxicology is required with special emphasis on testing available in community or regional hospital laboratories. The residents will demonstrate the following skills by the conclusion of the residency program: xvii. Given a clinical scenario, will provide appropriate advice 16 regarding biochemical test selection with a view to optimizing laboratory utilization. xviii. Will have a practical knowledge of statistics pertinent to clinical biochemistry. This will include the concepts of sensitivity, specificity, efficacy, precision, accuracy, incidence, prevalence, predictive value, reference ranges, means, standard deviation, variance, parametric and non-parametric distribution, and the control of pre-analytical variables. xix. Will demonstrate knowledge of common analytical techniques and instrumentation in the biochemical laboratory. xx. Will demonstrate an understanding of laboratory equipment selection. xxi. Will define the components of a quality assurance program and describe the methods of quality control and their application. xxii. Will demonstrate an understanding of the principles of laboratory safety and the regulations as they apply to workplace hazards and transportation of dangerous goods. xxiii. Will define the basic components of a Laboratory information system and its application to the modern biochemical laboratory. F. Medical Microbiology Medical Microbiology as applicable to General Pathology includes the following major areas of activity: The supervision and direction of the clinical microbiological laboratory at the level of the community or regional hospital. The direction of a hospital infection control program as it pertains to the role and utilization of the hospital laboratory. The provision of consultative services to clinical colleagues regarding appropriate microbiological investigations and their interpretation. The General Pathologist may be expected to assume some of the responsibilities of an infectious disease consultant depending on the availability of such resources and an understanding of common antimicrobial agents and their appropriate use if required. The organisms for which a working knowledge is required are those that are normally isolated or otherwise identified in a regional hospital laboratory. Bacteria: staphylococci, streptococci, Corynebacteriae (including other aerobic and facultative gram-positive rods), Clostridia, Neisseriae (including moraxella), Enterobacteriaceae, Campylobacter, Pseudomonas (and other common gram negative opportunistic bacilli), Hemophilus, Bordatellae, Legionellae, Chlamydiae, Mycoplasmae, and common pathogenic mycobacteria. Fungi: Candida, Aspergillus, Histoplasma, Coccidioides, Blastomyces, 17 Cryptococcus, Mucor, and Pneumocystis. Parasites: Malaria, ehrlichia, common helminthic infections (cestodes, Enterobius, Strongyloides, Ascaris), Giardia, Schistosomes, Cryptosporidia, Microsporidia, Entamoeba, Dientamoeba, blastocystis, echinococcus, Trichinella. For less common bacterial, fungal, and parasitic organisms there should be a general understanding of testing strategies, specimen collection and handling, laboratory safety, and interpretation of diagnostic reports. A basic knowledge of viral classification and identification techniques is required with particular emphasis on public health and hospital infection control implications. The residents must know the common bacterial toxins, associated disease implications, and toxin identification techniques. Hospital infection control must be understood in some depth including prevention and control of infection and epidemics, disinfection and sterilization procedures, appropriate handling and disposal of infectious materials, employee health and laboratory safety issues, and pertinent public health regulations. The residents must know the common quality control procedures applicable to microbiology. The residents at end of training must be able to demonstrate the following skills: iii. iv. v. vi. vii. viii. ix. x. xi. A practical knowledge of all common bench-level test methods including manual, semi-automated and automated systems. The ability to prepare and interpret Gram, Ziehl-Neelsen and special stains for fungi and parasites. The ability to interpret fluorescence microscopy. The ability to recognize the diagnostic features of common bacterial species on differential media, including common fermentation patterns. The ability to interpret culture data from non-sterile body sites, presuming knowledge of the common components of normal flora. The ability to describe the common etiological pathogens of infectious disease by disease process and body site. The ability to recognize common fungal and parasitic organisms in human tissue and to utilize serologic and culture investigations for diagnosis. The ability to utilize and interpret serological investigations for the diagnosis of bacterial and viral diseases. A working knowledge of the newer molecular diagnostic 18 methodologies and their use in microbiological diagnosis and outbreak investigation. xii. The ability to analyze and interpret antimicrobial sensitivity data and to describe specific techniques for assessing antimicrobial sensitivity. xiii. The ability to interpret quality control data applicable to Medical Microbiology. G. Hematopathology and Transfusion Medicine The level of expertise in hematology must reflect the knowledge base required in a regional or community hospital. The discipline as applicable to General Pathology includes the following major areas of activity: . i. ii. iii. iv. The supervision and clinical direction of a hematopathology laboratory as organized at the level of a regional or community hospital. The supervision and clinical direction of a transfusion service in association with provincial and national blood agencies. The morphological assessment and diagnosis of blood, bone marrow and lymph node based disorders with utilization of newer technologies as appropriate. The provision of consultation services with regard to appropriate and effective hematological investigation. The provision of consultation services regarding appropriate use of, and possible alternatives to, blood component therapy. The General Pathology residents must have a basic knowledge of the following: v. vi. vii. viii. ix. x. Normal hematopoiesis and cell biology as it pertains to the structure and function of all hematopoietic elements. The structure and functional relationships of all components of the reticulo-endothelial system. The components of humoral and cellular immunity, the role of complement and its pathways of activation. The components and functional relationship of the hemostatic and fibrinolytic systems including control mechanisms. Immunohematology including major blood group systems and the role of the human leukocyte antigen (HLA) system. Genetics as applicable to blood disorders. This knowledge base is required as a framework for understanding disorders of hematopoiesis and coagulation and to successfully resolve problems of blood component therapy. The knowledge base of hematopathological disorders must include the following: xi. Common anemias including diagnostic strategies, 19 xii. xiii. xiv. xv. xvi. xvii. xviii. morphological findings at the peripheral blood and bone marrow level, clinical associations, complications, and basic principles of management. Major causes of polycythemia including diagnostic strategies, morphological features, clinical associations, complications, and basic principles of management. Common non-neoplastic disorders of leukocytes including reactive, congenital and drug-related abnormalities. Common neoplastic disorders of leukocytes including diagnostic strategies, common classification schemes and the role of cytogenetics, stem cell studies, and flow cytometry. Major categories of lymphoma including common diagnostic strategies, morphological features, ancillary investigations, and prognostic features. Common disorders of thrombocytes including diagnostic strategies, clinical associations and principles of management. Major disorders of coagulation, congenital and acquired, including strategies for investigation, clinical associations and principles of management. Common problems of blood banking including incompatible cross-match, auto- and alloimmune antibodies and their differentiation, neonatal blood banking issues, types and investigation of adverse reactions to blood component therapy and the appropriate use of blood components in the treatment of hematological and coagulation disorders. At the end of training, the residents will have developed skills in the following: xix. xx. xxi. xxii. xxiii. xxiv. xxv. xxvi. Bench level tests available in a community or regional hospital hematology laboratory. This will include manual, semiautomated, and automated tests in addition to the basic principles of test methodology and instrumentation. Peripheral blood film and bone marrow/lymph node biopsy interpretation. This must include all abnormalities likely to be encountered in a community/regional hospital laboratory practice. Decision-making regarding appropriate use of newer diagnostic methodologies for hematological diagnosis. Constructing test strategies to diagnose common disorders of hematopoesis and coagulation. Bench level testing in the blood bank and recognition of standards as they apply to the testing and release of blood products. Assessing transfusion orders in relation to appropriateness, risks of blood product transfusion, and alternatives to transfusion. Transfusion reaction investigation. Hematology Quality Assurance (QA) and Quality Control (QC) issues and laboratory safety practices. 20 xxvii. Skill in bone marrow aspiration and biopsy technique should be acquired. Communicator General Requirements o o o o Establish therapeutic relationships with patients/families. Obtain and synthesize relevant history from patients/families/communities. Listen effectively. Discuss appropriate information with patients/families and the health care team. Specific Requirements Laboratory physicians, as active members of the health delivery team, will establish appropriate relationships with consulting physicians and surgeons. From time to time, especially in the clinical disciplines, the pathologist may find it helpful, in the best interests of patient care, to communicate directly with patients, families, and other health care providers. In this regard the residents must demonstrate skills in communicating, both in verbal and written form, in a manner appropriate to the intended recipient. The residents must understand effective clinical history taking and must have a broad knowledge of the laboratory basis of diagnosis in order to appropriately advise regarding test strategies and interpretation. Effective communication with clinical colleagues is essential in order to interpret surgical and autopsy pathology findings in the clinical context. The residents must be able to formulate comprehensive and clinically meaningful surgical pathology reports and organize diagnostic summaries to prioritize the features of importance. Diagnostic uncertainty must be clearly expressed with appropriate differential diagnoses and suggestions regarding further studies or ancillary investigations. There must be an awareness of ethical and medico-legal issues regarding the release and dissemination of confidential patient information. The residents must demonstrate awareness of the importance of timeliness, clarity and accuracy in all verbal and written communications. Collaborator General Requirements o o Consult effectively with other physicians and health care professionals. Contribute effectively to other interdisciplinary team activities. Specific Requirements The residents must be aware of the strong interface between the laboratory and clinical disciplines. The residents must also develop skills in supporting educational and/or research endeavours of clinical and laboratory colleagues through individual opportunities or group learning experiences. In the realm of surgical pathology the residents must be aware of, and respond appropriately to, situations in which the laboratory will significantly affect critical patient management decisions. Such situations will include intraoperative consultations, assessment of surgical margins, staging 21 procedures, situations where deferral of diagnosis is recommended, and situations where ancillary investigations or consultation is required for optimal case management. In the clinical disciplines the residents will be able to assist in optimal laboratory utilization appreciating the diagnostic limitations of laboratory tests and the importance of control of pre-analytic variables. The residents will understand the clinical requirements for turnaround time in specimen reporting, the range of testing which should be continuously available in the community/regional hospital and the appropriate laboratory response to critical values. The residents must understand the value of interdisciplinary and intradisciplinary collaboration in patient management decisions. This includes the need for case review including review by external institutions and agencies. The residents must demonstrate a willingness to seek consultation opinions if so requested by clinical colleagues with modification of subsequent diagnostic impressions if appropriate. Manager General Requirements o o o o Utilize resources effectively to balance patient care, learning needs, and outside activities. Allocate finite health care resources wisely. Work effectively and efficiently in a health care organization. Utilize information technology to optimize patient care, life-long learning and other activities. Specific Requirements At the end of training, the residents will understand the basic principles of laboratory management. Specifically the residents will have some knowledge of: o o o o o o o o o o o o o Staffing and personnel management. Budgeting (personnel, materials, capital equipment) Workload measurements. Funding structures for laboratories. Hospital medical staff organization and roles. Quality control, quality assurance and continuous quality improvement. Laboratory safety and the transportation of dangerous goods. Management styles. Principles of optimal laboratory utilization. Equipment purchasing and selection. Relevant legislation and/or regulations governing the operation of laboratories, including issues of informed consent. Relevant legislation and/or regulations governing laboratory operation and informed consent Laboratory information systems and components (hardware and software). It is expected that management issues pertinent to the laboratory will be taught as part of the academic activities of the residency-training program and may be supplemented with specific research activities. 22 Health Advocate General Requirements o o o Identify the important determinants of health affecting patients. Contribute effectively to improved health of patients and communities. Recognize and respond to those issues where advocacy is appropriate. Specific Requirements As part of an interdisciplinary team of professionals responsible for patient and community health care, the residents will understand those components of the laboratory and its services that are required to: iv. Respond adequately to community, and hospital service demands including the need for population screening. Respond to hospital, community and regional public health needs to detect and control infectious disease. Provide sufficient and safe blood bank resources. v. vi. The residents will demonstrate the ability to recognize and respond to situations where health advocacy and application of health care resources is required. This will include the introduction of improved instrumentation and methodologies to augment community health care. Scholar General Requirements o o o o Develop, implement and monitor a personal continuing education strategy. Critically appraise sources of medical information. Facilitate learning of patients, housestaff/students and other health professionals. Contribute to development of new knowledge. Specific Requirements During the training period, the residents will demonstrate an ability to develop and implement a strategy for learning including a program of continuing education following completion of the residency. There must be a working knowledge of statistics applicable to all aspects of laboratory medicine and the capability of appraising sources of medical information. An important aspect of the role of the general pathologist is the continuing education of laboratory technologists and clinical medicine colleagues. An ability to perform this educational role must be developed by the senior residency years. Contribution towards new knowledge is a major role of academic laboratory medicine specialists and the General Pathology residents must also be familiar with research methodology. In this regard the residents should have undertaken at least one research project during the 5-year program and must be familiar with the principles of critical appraisal. Professional General Requirements 23 o o o Deliver highest quality care with integrity, honesty and compassion. Exhibit appropriate personal and interpersonal professional behaviours. Practise medicine ethically consistent with obligations of a physician. Specific Requirements The residents must develop a broad understanding of the role of the physician within the community and hospital structure. The residents must establish a high standard of laboratory medical practice, appreciating personal limitations in diagnostic skill which will require referral of particular types of case in the best interests of patient care. The residents will demonstrate integrity, honesty and compassion in all aspects of the practice of laboratory medicine as well as personal and interpersonal professional behaviours of a high ethical standard. These behaviours will include those relating to confidentiality, respect for others, conflict of interest, codes of conduct, personal and professional boundaries, consent, and the role of professional self-regulation and continuing education. Specialty Training Requirements Editorial revision — November 2002 (These specialty training requirements apply to those who began training on or after 1 June 2000. Please see also the 1994 training requirements pertaining to those who began training on or before 31 May 2000.) Five years of approved residency. This period must include: 1. One year of basic clinical training. 2. a. Two years of approved training in anatomical pathology; these two years must include: i. ii. iii. at least one year of surgical pathology; at least 2 months of training in a formal, structured medico-legal autopsy program, which must provide exposure to an appropriate mix of medico-legal autopsy cases in sufficient numbers to meet training objectives; 3 months training in cytopathology. b. Six months of approved training in each of the following: i. ii. iii. medical biochemistry; hematological pathology, incorporating morphological hematology, coagulation, and transfusion medicine; microbiology, including bacteriology, immunology, mycology, parasitology and virology. c. One of the following options. These should include experience in laboratories in smaller community hospitals and to freestanding clinical laboratories. It must include training in facilities with laboratory physicians practicing general pathology. 24 i. ii. iii. Up to six months of approved residency training in a university affiliated hospital, preferably community based, integrating the skills required of a general pathologist in a practice setting. Six months of further approved training in one or more of the following: anatomical pathology, medical biochemistry, hematological pathology, or microbiology. Six months of other approved residency or research, relevant to the objectives of general pathology and acceptable to the director of the training program, at a hospital or university centre in Canada. 25 Further to the RCPSC objectives are the program’s general objectives: GENERAL OBJECTIVES 1. Medical Expert A thorough knowledge of cellular, tissue and organ structure, function, and pathophysiology as they apply to human disease. An adequate knowledge of practical clinical problems to enable the graduate to identify and fulfill clinical service needs and to make useful clinicopathological correlations. A knowledge of laboratory safety precautions within the department (e.g. handling of infectious cases). A "safe" level of skills in surgical pathology and biopsy diagnosis, combined with an understanding of your own limitations and when and how to obtain assistance from more experienced colleagues. A full range of skills in general and special autopsy techniques, including medico-legal autopsies. A full range of skills in surgical pathological techniques, including rapid diagnosis on frozen sections, immunohistopathology, and molecular pathology. A full range of skills in exfoliative cytology and aspiration cytology diagnosis, and screening cytology. Special skills in selected subspecialty areas. A full range of skills in Hematopathology including blood banking, coagulation, instrumentation, and peripheral smears and bone marrows. The resident will learn how to obtain bone marrow aspirates and trephine biopsies. A full range of skills in medical biochemistry including electrophoresis, screening and toxicology. A full range of skills in medical microbiology including virology, mycology, bacteriology, parasitology and molecular technology. An ability to take photographs of gross and microscopic specimens. 2. Communicator A knowledge of the roles of the Anatomical Pathologist in medical audit and the generation, communication and storage of data. The ability to generate clear, concise, comprehensive and accurate written pathology reports. 3. Collaborator An understanding of the role of the Anatomical Pathologist as a member of a health care team, including understanding of the ways in which the roles of the Anatomical Pathologist differ from, as well as complement, those of other members of the team. 26 4. Manager A sound knowledge of the technical basis of Anatomical Pathology as a medical specialty, so that you can effectively organize and direct a laboratory. A sound knowledge of the principles and practices of quality assurance and quality control in Anatomical Pathology. Acquire the ability to manage the day-to-day work of an anatomical pathologist in order to deliver high quality laboratory/pathology results in a timely manner to optimize patient care. 5. Scholar Recognition of the essential nature of accurate professional self-assessment and of continuing education and honing of skills. Responsibility to keep abreast of recent advances in Anatomical Pathology on an ongoing basis, through a diligent coverage of journals, monographs, symposia, and attendance at meetings and workshops. An ability to make presentations at educational/teaching rounds (departmental and interdepartmental). A knowledge of the role of the Anatomical Pathologist in medical education. A commitment to participate in undergraduate, graduate and post-graduate medical education. Contribute to the development of new knowledge through research. 6. Professional Deliver the highest quality of care with integrity, honesty, and compassion. Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner. Practice medicine in an ethical manner and with sensitivity to diverse patient and co-worker populations. Recognition that every Anatomical Pathologist, no matter how competent, needs the assistance of his colleagues to help him keep his errors to a minimum and recognition that he owes his help to his colleagues in turn. Understanding that the practice of Anatomical Pathology is a profession and not a business. 7. Health Advocate Recognition that the responsibility of an Anatomical Pathologist in hospital practice is primarily for the welfare of the patient. Recognition that all Anatomical Pathologists make some errors and that these must never be glossed over or covered up, but must be brought out and corrected and studied in order not to be repeated. Learn how to effectively educate and advise clinical/surgical practitioners/colleagues on the appropriateness and quality of diagnostic tests to optimize patient care. 27 CONTENT AND ORGANIZATION OF THE PROGRAM Content and Sequence of Training PGY1: (weeks) 4 - ER; 8 - Elective; 4 - Psychiatry; 4 - Pediatrics; 4 - Radiation Oncology; 4 - General Surgery; 8 - Internal Medicine; 4 - CCU; 4 - Diagnostic Radiology; 4 – Family Medicine PGY2: 8 weeks AP @ UAH 2 weeks autopsy path @ MEO 1 week histo lab UAH 13 weeks AP @ UAH 24 weeks @ 2 clinical rotations PGY3: 12 weeks @ 1 clinical rotation 6 weeks AP @ DynaLIFE 6 weeks cytology 1 @ UAH/DynaLIFE 4 weeks breast/GU @ MIs (to be taken before surg path rotation) 4 weeks lymphoma @ CCI (to be taken before molecular path rotation) 4 weeks neuropathology (Oct – Dec only) 4 weeks AP @ GNH 8 weeks elective PGY4: 2 weeks dermpath @ UAH 24 weeks @ 2 clinical rotations 8 weeks @ MEO 12 weeks surg path/gyne path @ RAH 2 weeks diagnostic molecular path PGY5: 2 weeks diagnostic molecular path 4 weeks surg path @ MIS 12 weeks @ 1 clinical rotation 6 weeks cytology 2 @ UAH/DynaLIFE 20 weeks GP @ DynaLIFE 4 weeks study/exam leave 28 ACADEMIC AND SCHOLARLY ASPECTS OF THE PROGRAM Half-day Teaching Schedule Current schedule available on Webeval site (https://www.webeval.med.ualberta.ca/webeval/index.php). Other regular teaching sessions/clinical rounds Other regular teaching sessions/clinical rounds CME Rounds at DynaLIFE (weekly) Department of Laboratory Medicine and Pathology Grand Rounds (weekly) Department of Medicine Grand Rounds (weekly). Dermatopathology Rounds (bi-monthly). G.I. Pathology Rounds (weekly). General AP teaching session (includes Cytology) (weekly) General Pathology Journal Club (monthly). GI Clinical Conference (weekly). Neuropathology Rounds (weekly). Pulmonary Cytology Rounds (weekly). Radiology/Pathology Rounds (monthly). Renal Pathology Rounds (bi-monthly). Soft Tissue teaching session (bi-monthly). Occasional teaching sessions/clinical rounds Head & Neck Pathology Rounds Surgery A Pathology Rounds Surgery B Pathology Rounds 29 RESEARCH IN THE GENERAL PATHOLOGY RESIDENCY PROGRAM The Royal College of Physicians and Surgeons of Canada have established the following terminal objectives in the Scholar domain of their Objectives of Training and Specialty Training requirements in General Pathology document: “During the training period, the residents will demonstrate an ability to develop and implement a strategy for learning including a program of continuing education following completion of the residency. There must be a working knowledge of statistics applicable to all aspects of laboratory medicine and the capability of appraising sources of medical information. An important aspect of the role of the general pathologist is the continuing education of laboratory technologists and clinical medicine colleagues. An ability to perform this educational role must be developed by the senior residency years. Contribution towards new knowledge is a major role of academic laboratory medicine specialists and the General Pathology residents must also be familiar with research methodology. In this regard the residents should have undertaken at least one research project during the 5-year program and must be familiar with the principles of critical appraisal.” Residents in General Pathology are expected to identify a research project either during their PGY1 year or early in their PGY2 year. The faculty member responsible for coordinating resident research (Dr. Victor Tron) is available to assist residents in the selection and implementation of a reasonable research project. The projects may be either basic or clinical in nature. It is expected that residents will make one presentation at the Resident Research Day during their residency. The Department of Laboratory Medicine and Pathology holds an annual Resident and Graduate Student Research Day in conjunction with the annual Macgregor Memorial Lecture usually held each fall. The external judge is the John W. Macgregor Lecturer for the year and a prize is awarded for the best resident research presentation. Approved November 2001 30 RESIDENT GRADED RESPONSIBILITY IN GENERAL PATHOLOGY Anatomical Pathology: Graded responsibility is addressed from the point of view of clinical service and education. Both program organization and the level of expectation by staff, of a resident’s performance at different levels of training are the principal mechanisms by which graded responsibility is implemented. For example, during the PGY2 year (first year of pathology training) the resident is exposed to a broad spectrum of basic gross and microscopic surgical and autopsy pathology. In contrast, during the final year of training, senior residents participate in a variety of elective rotations which concentrate on more sophisticated levels of histopathological evaluation in subspecialized areas of anatomical pathology. They also have an opportunity to review substantial volumes of consultation material from the various staff members with a variety of subspecialty interests. During the first two years of training, the expectation of residents is that they should comprehend basic gross and microscopic pathology, gain expertise in dissection, become familiar with laboratory techniques in general and appreciate the significance of clinico-pathologic correlation. At this level of training, they are not expected to report surgical cases routinely (but may be assigned simple cases for signout) and their participation in frozen section pathology is primarily as observers. As training proceeds, residents will be given increased responsibility in preparing surgical reports and to participate more actively in handling frozen section cases. During the last two years of training, residents are expected to have a comprehensive approach to the interpretation of complicated cases, be adept at communication with clinicians and to participate actively in educational activities directed at junior residents and medical students. In addition, they are required to generate comprehensive pathology reports of high quality on a routine basis and are expected to document their diagnoses at the time of frozen section prior to reporting by a staff member. Hematological Pathology: At the beginning of his/her Hematopathology (HP) rotation at the UAH site, the General Pathology (GP) resident is taught to approach morphology systematically and throroughly. Initially, his/her primary focus in morphology is on the review of abnormal blood smears referred in by the senior technologists (with senior Hematopathology staff back-up whenever required), and on reading of peripheral smears and bone marrows alongside the hematopthologists signing out the cases for the day. As the GP resident gains more experience in HP morphology, he/she is encouraged and expected to dictate his/her own morphology reports, starting with peripheral smears and progressing on to bone marrows and cell marker reports. These reports are always reviewed and co-signed by a hematopathologist. During his/her HP rotation, the GP resident is also taught to perform bone marrow aspirations and biopsies. Initially (usually for the first 2 - 3 weeks), the staff hematopathologists supervise the procedures very closely, but as the resident gains experience and confidence, he/she will graduate to performing the procedure on his/her own. Senior staff back-up is always available. A similar process if assuming more responsibility with time and experience is in place for other areas of HP, and the resident handles problems from these areas on call with senior staff back-up. The rate at which a resident gains increasing autonomy obviously depends on the individual concerned, but the expectation is for the GP resident to be able to handle with competence most of the common hematology issues seen in general hospital laboratories at the end of 6 months of HP training. 31 In all areas, as the GP resident becomes more experiences, he/she also undertakes a supervisory role with respect to more junior residents and elective medical students. Medical Biochemistry: General Pathology residents spend a total fo 24 weeks in Medical Biochemistry, 12 weeks during the second and third years and 12 weeks during the fourth and fifth years. The first rotation focuses on introduction to clinical chemistry with emphasis on blood collection, sample handling, analytical techniques, general biochemistry and quality assurance. The resident is on-call after the first two weeks of the rotation and with the help of a staff person is expected to handle calls regarding appropriate test requests, and interpretation of results. During the second rotation more emphasis is placed on specialist areas although the resident does receive revision of the areas covered in the first rotation. The resident is expected to have a greater depth and breadth of knowledge in laboratory medicine and is encouraged to have a greater consultative role. The specialist areas provide more opportunity for interaction with physicians and the resident is encouraged to call physicians with request for further patient information e.g. with reference to toxicological testing and examine patient charts if necessary. The resident is also expected to have sufficient knowledge eto discuss more esoteric test results and suggest further appropriate further testing. Medical Microbiology: General Pathology residents-in-training spend two twelve-week blocks in Medical Microbiology. Most of that time is spent in the Medical Microbiology Laboratory at University of Alberta Hospital. Additional time is spent at DynaLIFE during the last 24 weeks of their program when they may also have responsibilities in medical microbiology as well as other disciplines. During the first 4 weeks of the first 12-week rotation in Medical Microbiology at UAH, the resident is not on call. This permits them time to familiarize themselves with the day-to-day functions of the laboratory and to re-acquaint themselves with the fundamentals of medical microbiology. The last 8 weeks they are inserted into the regular resident call rotation along with medical microbiology residents in the department. The microbiologist on call is always available for backup, and decisions made by the resident are discussed with the microbiologist on call as a continuing learning exercise. The resident is encouraged during this period to make regular daily decisions on interpretation at the bench level during daily rounds and to interact with the microbiologist on call on interpretation of significant results. During the second 12-week rotation, the resident is included in the full rotation of on call schedules for residents. They take first call for the laboratory, but still always have a microbiologist on call as backup. Once again the resident interactions with clinicians are discussed with the microbiologist, and they are involved in the routine sign-out of significant reports from the laboratory. In this way, graded responsibility is developed so that by the end of their training the resident can function actively and confidently with clinicians in the clinical interpretation of microbiology results. 32 ROTATION SPECIFIC OBJECTIVES MANDATORY ROTATIONS 33 ROTATION SPECIFIC OBJECTIVES: AUTOPSY PATHOLOGY TECHNIQUE Selection: Site: Preceptor: Length of Rotation: Prerequisites: Specific Objectives: Mandatory Medical Examiner’s Office Chief and Assistant Chief Medical Examiner Two weeks PGY-1 MEDICAL EXPERT Learn the technical aspects of performing an autopsy and perform an autopsy from the opening incision to the closing suture for both adult and paediatric cases. Learn variations of autopsy technique (i.e. block versus organ-by-organ) and special autopsy procedures (eg. neck dissection, leg vein dissection, eye removal, collection of appropriate blocks for cardiac conduction system examination, collection of blood cultures). Acquire skills in gross examination of organs and interpretation of gross organ pathology at a level appropriate to this early stage of training. Perform a complete and appropriate assessment of a patient. Become familiar with the difference in consent procedures required for conducting a hospitalbased family-consented autopsy versus a medicolegal autopsy. Begin learning the types of questions an autopsy cannot answer. Become familiar with normal postmortem histology. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Summarize the medical history and scene findings for the staff pathologist, summarize the questions that must be answered by an examination of the body, decide whether an autopsy is required in order to answer those questions, and justify the reasoning for the choice of examination type. Provide the staff pathologist with a differential for the expected autopsy findings/cause of death and explain how that differential impacts the choice of autopsy technique and/or the use of special autopsy procedures. Present gross anatomic findings to the staff pathologist at the completion of every autopsy. Become familiar with the contents of an autopsy report in preparation for dictating these reports independently. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). 34 Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. 35 COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Respond to the individual health needs of the patient and their family and issues as part of patient care, including specific needs centered on the end of life issues. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop an individualized case-by-case approach to the conduct of an autopsy, based upon answering relevant questions that the clinical history and circumstances of the death raise. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Show respect for the deceased at all times. Know and follow all safety precautions in the autopsy facility, and understand the role a pathologist must play in ensuring the safety of all morgue staff and any observers at an autopsy. Develop an understanding of varying religious, ethnic, and cultural beliefs about the autopsy and the preparation of a body for ultimate disposal. Develop an understanding of how the autopsy technique can impact a funeral home’s preparation of the body for viewing. Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. 36 Outline of Rotation: The resident will review the charts of all cases requiring examination, first thing every morning of the rotation. The resident will summarize the cases to the staff pathologist, explain which cases require an autopsy, and why an autopsy is required. For those cases requiring an autopsy, the resident will provide a differential of the most likely causes of death, will explain whether any cause of death in the differential requires modifications of the basic autopsy technique, and whether any special autopsy procedures will be needed. The resident will perform all technical aspects of the autopsy on both adult and paediatric nonhomicide cases, to a maximum of two autopsies per day, including: opening incision; removal of chest plate with rib cutters; collection of toxicology specimens; evisceration of the neck, thoracic, abdominal, and pelvic organs; removal of the brain, including use of an oscillating bone saw and dura stripper; examination of all organs with collection of appropriate tissue blocks; return of viscera to body; and closure of body. The resident will stop the autopsy immediately upon coming across an unexpected finding, will present the finding to the staff pathologist, will discuss how the autopsy technique should be modified in order to best demonstrate and document the finding, and will then proceed to modify the technique in accordance with the outcome of that discussion. The resident will demonstrate to and discuss the gross anatomic findings with the staff pathologist at the completion of each autopsy. At the conclusion of every autopsy, the resident will outline and discuss what additional tests (eg. histology, neuropathology, toxicology, etc.) they would conduct in order to answer relevant outstanding autopsy questions. The resident will participate in performing special autopsy procedures as cases permit. The resident will learn and observe all safety rules while conducting postmortem examinations. Educational Materials: 1. 2. 3. Ludwig J. Handbook of Autopsy Practice 3rd Edition. Humana Press, Totowa: 2002. Boglioli LR and Taff ML. Religious Objection to Autopsy: An Ethical Dilemma for Medical Examiners. Am J Forensic Med Pathol 11: 1-8, 1990. Sternberg S. Histology for Pathologists 2nd Edition. Lippincott-Raven Publishers, Philadelphia: 1997. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 37 ROTATION SPECIFIC OBJECTIVES AUTOPSY AND SURGICAL PATHOLOGY 1. Medical Expert/Clinical Decision-Maker Ability to conduct appropriate gross examination and dissection of surgically excised tissues including: - examination of arterial supply and venous drainage - examination of regional lymph nodes - selection of appropriate tissue blocks for histological study - preparation and examination of quick frozen sections, when indicated Knowledge of the Fatality Inquiries Act and laws or other statutes governing death, organ donation, burial and the performance of autopsies. Ability to perform a complete autopsy on an adult patient, including the evisceration, dissection and blocking of organs. Skill in interpretation of microscopic pathology to enable identification of common disease processes and formulation of a reasonable differential diagnosis for less common conditions. Ability to satisfactorily create a full autopsy report including an accurate clinical summary, description of gross and microscopic findings, clinico-pathologic correlation, final diagnosis(es) and cause of death (if possible). Ability to describe and recognize grossly and microscopically the common lesions examined in the routine surgical service of a medium-sized hospital and to use the appropriate consultant options. Ability to effectively supervise the routine technical procedures of the histology laboratory and troubleshoot any QA/QC issues that arise.. Familiarity with the principles of tissue fixation and processing, common special staining procedures i.e. neutral fat, glycogen, elastin etc., and immunohistochemistry with selection of appropriate stains and panels. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Integrate clinical, radiological and other laboratory data to provide the best diagnosis and direct further investigations and therapeutic strategies. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. 2. Communicator Assist in the continuing education of physicians and other members of the hospital staff by participating in conferences and case presentations. Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient and to assist in further diagnostic studies if samples are insufficient for diagnosis. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. 38 Develop a common understanding on issues and problems with colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. 3. Collaborator Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens for diagnostic, teaching and research purposes and to advise on further appropriate investigations. Contribute effectively to interdisciplinary team activities by participating in interdisciplinary rounds or research activities. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. 4. Manager Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life. Allocate finite health care and health education resources effectively to optimize patient care and life-long learning. Work effectively and efficiently in a medical laboratory organization. Familiarity with quality control procedures in histology. Familiarity with methods of tissue collection for receptor studies and staining by immunological methods. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. 5. Health Advocate Identify the important determinants of health affecting patients pertaining to disease processes. As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs. Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge and become aware of one’s own diagnostic limitations/thresholds to ensure patient safety, and accuracy of medical/pathology reports. 39 6. Scholar Develop and implement a personal continuing educational strategy. Apply the principles of critical appraisal to sources of medical information. Contribute to the development of new knowledge through research. Participate in rounds, conferences and teaching sessions. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. 7. Professional Deliver the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethical manner and with a sensitivity to diverse patient and coworker populations. Exhibit appropriate professional behavior and perform duties in a dependable, consistent and responsible manner. Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: In the early phase of training, the trainee learns the basic techniques and skills required for the performance of autopsies in all categories of cases. He must learn the discipline of completeness and thoroughness; he must learn to make accurate and meaningful observations and to accurately and completely record his findings. This requires example, supervision and practice. The resident is on the autopsy service on a rota basis and does a minimum of every third autopsy. The resident is to check with Admitting and/or the Autopsy Assistant to determine if there is an autopsy for that day. If there is, the resident must ensure that all documentation is appropriate, notably that the consent for the autopsy is appropriate and determine if the case needs review by the MEO. The resident needs to inform the responsible staff about the autopsy. The resident must review the chart and present a thorough summary of the case to the staff pathologist. The resident will discuss the best approach to the case with the staff and will only proceed with the autopsy with the approval of the staff involved. After the resident has completed the two week autopsy rotation at the MEO office, he/she is expected to produce a completed protocol for each of his autopsy cases based upon his gross and microscopic observations and the details of the clinical record. His protocol includes a summary which outlines the major clinical and pathological findings, details of the clinicopathological correlations and identifies any unresolved problems. The compiled protocol includes drawings, photographs, descriptive material and microbiological reports in addition to weights and measures, clinical summary (including therapy), 40 pathological diagnosis and final summary with clinicopathological correlations. The resident must formulate a preliminary pathological diagnosis and present it to the staff. The resident must finalize the case in a timely fashion. This is particularly important if the resident is to leave the service. The resident will cover a subspecialty area one week at a time. During this week, they are to macroscopically and microscopically examine all specimens pertaining to the subspecialty area covered. To ensure adequate grossing experience, the rota will cover high volume subspecialty areas (head & neck, large GI, GU) more frequently with lower volume subspecialty areas covered less frequently. The resident will also cover frozen sections on every fourth day. Call coverage will be one week at a time, from home, shared with all other PGY2-5 residents. The resident will be expected to preview cases that they have examined macroscopically, formulate a differential diagnosis, order ancillary tests and issue microscopic reports according to the skill level of the resident. The resident should strive to acquire accuracy, efficiency, breadth and speed in handling the surgical routines. The resident is required to read around cases and perform literature searches. The resident is also encouraged to maintain a regular schedule of standard textbooks to ensure that all topics are covered thoroughly. Educational Materials: Suggested reading: Robbins Pathologic Basis of Disease, 6th edition, W.B. Saunders Company, 1999. Ackerman’s or Sternberg. Ludwig’s Handbook of Autopsy Practice, 3rd edition. Clinical material: Current cases Archival material (Resident Teaching File) Wheater’s Basic Histopathology, 4th edition. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. May 2007 41 ROTATION SPECIFIC OBJECTIVES FOR BREAST/ MALE GENITAL AND URINARY PATHOLOGY Selection: Site: Preceptor: Length of Rotation: Specific Objectives: Mandatory Misericordia Hospital Dr. J. Danyluk Four weeks MEDICAL EXPERT To develop expertise in optimal intraoperative and gross examination of breast specimens, utilizing specimen x-ray for maximum diagnostic information. To have knowledge of and be able to diagnose and report most benign and malignant abnormalities in the breast and to have knowledge of the pitfalls. To have knowledge of and be able to diagnose and report most benign and malignant abnormalities in the urinary bladder and prostate as they present in transurethral resection, endoscopic and needle biopsies, and to have knowledge of the pitfalls. To have knowledge of and be able to diagnose and report most of the neoplasms and pseudotumors in the testis, scrotum and spermatic cord, and to have knowledge of the pitfalls. To examine ethical and quality assurance issues that might arise as case material is examined. To expand an appreciation of the role of the pathologist as a clinical consultant in the multidisciplinary setting. function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care establish and maintain clinical knowledge, skills and attitudes appropriate to their practice perform a complete and appropriate assessment of a patient use preventative and therapeutic interventions effectively demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic seek appropriate consultation from other health professionals, recognizing the limits of their expertise Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Assist in the continuing education of physicians and other members of the staff by participating in conferences and case presentations. Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient. develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) 42 accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals accurately convey relevant information and explanations to colleagues and other professionals develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families convey effective oral and written information about a medical encounter Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens and to advise on further appropriate investigations. participate effectively and appropriately in an interprofessional healthcare team effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life. Allocate finite health care and health education resources effectively to optimize patient care and life-long learning. participate in activities that contribute to the effectiveness of their healthcare organizations and systems manage their practice and career effectively allocate finite healthcare resources appropriately serve in administration and leadership roles, as appropriate Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to pathological processes. As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs. 43 Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. respond to individual patient health needs and issues as part of patient care respond to the health needs of the communities that they serve identify the determinants of health of the populations that they serve promote the health of individual patients, communities and populations acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy. Apply the principles of critical appraisal to sources of medical information. Contribute to the development of new knowledge through research. Participate in rounds, conferences and teaching sessions. maintain and enhance professional activities through ongoing learning critically evaluate information and its sources, and apply this appropriately to practice decisions facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate contribute to the creation, dissemination, application, and translation of new medical knowledge and practices Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethnical manner and with a sensitivity to diverse patient and co-worker populations. Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner. Demonstrate commitment to excellence and ongoing professional development. demonstrate commitment to excellence and ongoing professional development demonstrate a commitment to their patients, profession, and society through ethical practice demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation demonstrate a commitment to physician health and sustainable practice Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. 44 Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: The resident shall be able: To participate in the intraoperative assessment of breast carcinomas. To perform the gross examination of breast resections for carcinoma and to effectively utilize specimen x-ray. To perform a microscopic examination and prepare surgical pathology reports relating to breast and small specimen urologic pathology specimens. To attend group meetings at the Misericordia Hospital. As time permits, examine case material referred by other pathologists and archived case material. Attend multidisciplinary breast rounds at the Cross Cancer Institute Educational Materials: Suggested reading: - Surgical pathology and specialty textbooks. - Collection of recent articles and literature. - The resident is expected to develop and follow a reading schedule, eg. Week 1 - Breast Examination, Benign and DCIS. Week 2 - Prostate Week 3 - Breast - Other Malignancies Week 4 - Urinary Bladder, Testis, Scrotal Contents and Spermatic Cord Conferences: 1. Surgical rounds at the Misericordia Hospital - to attend and present the pathology cases. 2. To attend and present at the Misericordia Breast Interest Group meeting. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 45 ROTATION SPECIFIC OBJECTIVES IN CYTOPATHOLOGY 1 @ UAH Selection: Mandatory. Site: University of Alberta Hospital. Preceptors: Dr. P. Makarla. Length of Rotation: Four weeks. Prerequisites: Twelve months autopsy pathology and general surgical pathology. MEDICAL EXPERT To become familiar with the organization and administration of a cytology laboratory, including quality assurance. To know how to handle different types of specimens from various body sites and the preparation method most suitable for the test required as well as fixation and staining techniques. To be able to recognize the cytological appearance of normal (exfoliated, scraped or aspirated) cells and the morphological differences between various cell types. To become familiar with cytological diagnostic criteria, limitations of cytology, and criteria of adequacy. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Eestablish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a case. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans with colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter and direct/advise further testing as the need arises. 46 COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Manage time to maximize educational resources and opportunities. Manage workload appropriately to ensure timely completion of case work. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Acquire appropriate QA/QC knowledge and regulations specific to cytology to ensure patient safety and accuracy of medical reports. Become fully cognizant of limitations in cytopathology to direct/advise clinicians on the further testing/management for a patient. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. 47 Outline of Rotation: The resident must meet with the preceptor at the beginning of the rotation. The resident should tour the UAH Cytology Department and familiarize himself with general process and relevant personnel. The resident will spend a minimum of two days in the laboratory area to understand, in depth, the principles and practices of specimen collection and preparation and the principles and rationales for use of various staining procedures. The resident should by reading and discussion develop an understanding of the appropriate role for cytologic investigations. On a daily basis, the resident will spend a minimum of one hour with Ms. Gray to become familiar with the cytological appearance of normal cells and to determine specimen adequacy determination for different specimen types. The resident will attend and assist at the weekly FNAB clinic run by faculty cytopathologists. The resident will thoroughly screen ten specimens to gain an appreciation for normal cellular components and to develop appropriate screening habits. The resident will actively participate on a daily basis with case sign-out to as great an extent as possible. The resident will become familiar with the range of Quality Assurance activities incorporated into the practice of cytology. Educational Materials: Required reading 1. Cibas, E.S. and Ducatman, B.S. Cytology: Diagnostic Principles and Clinical Correlates, W.B. Saunders Company, Philadelphia, 1996. 2. Orell, S.R., Sterrett, G.F., Walters, M.N-I., and Whitaker, D. Manual and Atlas of Fine Needle Aspiration Cytology, 2nd edition, Churchill Livingstone, Edinburgh, 1992. 3. Kurman, R.J. and Solomon, D. The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses. Springer-Verlag, New York, 1994. Suggested supplemental reading – 1. DeMay RM. The Art and Science of Cytopathology, ASCP, Chicago, 1996. 2. Koss, L.G. Diagnostic Cytology and Its Histopathologic Bases, 4th edition, J.B. Lippincott Company, Philadelphia, 1992. 3. Bibbo, M. Comprehensive Cytopathology, 2nd edition, W.B. Saunders Company, Philadelphia, 1997 Conferences – Pulmonary Rounds. Clinical material – 1. Current cases. 2. Archival material (Please consult with cytopathologists re: personal collections and Ms Gray re: Cytotechnology Student Teaching files. 3. FNA clinic. 48 Evaluation: 1. On completion of both this rotation and the 2-week rotation at DynaLIFE, the resident will be evaluated on his/her ability to adequately screen, in a timely fashion, 20 cases from different sites. The assessment will be done by Ms. Gray. 2. Overall assessment based on standard resident evaluation form provided by Program Director’s Office. 3. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Sept 2008 49 ROTATION SPECIFIC OBJECTIVES IN CYTOPATHOLOGY 1 @ DynaLIFE Selection: Mandatory. Site: DynaLIFE DX Diagnostic Laboratory Services Core Laboratory. Preceptor: Dr. G. Johnson. Length of Rotation: Two weeks. Prerequisites: Cytopathology 1 @ UAH MEDICAL EXPERT To be able to recognize common pathologic conditions in both gynecological and nongynecological cytology. To recognize common pitfalls in cytopathology. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient’s medical history relevant to diagnostic pathology. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals verbally and in the form of written cytopathology reports. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. COLLABORATOR Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens and to advise on further appropriate investigations. Collaborate with colleagues at all levels in the pathology laboratory particularly with respect to consultation, peer review and continuing professional development. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage time to maximize educational resources and opportunities. Manage workload appropriately to ensure timely completion of case work. Develop skills in the management of technical and support staff in the laboratory. Manage their practice and career effectively. Allocate finite healthcare resources and health education resources effectively to optimize patient care and life-long learning. 50 Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to pathological processes. Acquire appropriate QA/QC knowledge specific to cytopathology to ensure patient safety and accuracy of medical reports. Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. SCHOLAR Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices by participating in research, rounds, conferences and teaching sessions. Develop and implement a personal continuing education strategy. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. PROFESSIONAL Develop the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethical manner and a sensitivity to diverse patients and co-worker populations. Exhibit appropriate professional behaviour and perform duties in a dependable and responsible manner. Demonstrate commitment to evidence-based medicine, excellence, and on-going professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice/ Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. 51 Outline of Rotation: 1. Tour DynaLIFE Cytology Department and familiarize yourself with general process and relevant personnel. 2. One-hour session with Dr. Johnson or delegate to familiarize resident with reporting formats and protocols in place at DynaLIFE, including synoptic reports in use. 3, Understand, in depth, principles and practices of specimen collection and preparation. 4. Understand principles and rationales for use of various staining procedures. 5. Develop understanding of appropriate role for cytologic investigations. 6. Develop theoretical knowledge of fine needle aspiration techniques in various organ sites. 7. Understand specimen adequacy determination for both gyne and non-gyne specimens. 8. Thoroughly screen five of each specimen type to gain appreciation for normal cellular components. 9. Develop appropriate screening habits. 10. Actively participate on a daily basis with case sign-out to as great an extent as possible. 11. Understand and apply management recommendation for gyne cytology cases as outlined in “Alberta Standardized Management Recommendation” report. 12. Prepare for a 30-45 minute tutorial on non-gyne cytology each week during the rotation. 13. Prepare 30 minute CME session for presentation at biweekly cytopathology department conference. Educational Materials: Required reading 1. Cibas, E.S. and Ducatman, B.S. Cytology: Diagnostic Principles and Clinical Correlates, W.B. Saunders Company, Philadelphia, 1996. 2. Orell, S.R., Sterrett, G.F., Walters, M.N-I., and Whitaker, D. Manual and Atlas of Fine Needle Aspiration Cytology, 2nd edition, Churchill Livingstone, Edinburgh, 1992. 3. Kurman, R.J. and Solomon, D. The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses. Springer-Verlag, New York, 1994. Suggested reading – 1. Koss, L.G. Diagnostic Cytology and Its Histopathologic Bases, 4th edition, J.B. Lippincott Company, Philadelphia, 1992. 2. Bibbo, M. Comprehensive Cytopathology, 2nd edition, W.B. Saunders Company, Philadelphia, 1997 52 Clinical material – 1. Current cases. 2. Archival material (Please consult with Dr. Johnson). Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Sept 2008 53 ROTATION SPECIFIC OBJECTIVES IN CYTOPATHOLOGY 2 @ UAH Selection: Mandatory. Site: University of Alberta Hospital. Preceptors: Dr. P. Makarla. Length of Rotation: Four weeks. Prerequisites: Cytopathology 1 @ UAH; Cytopathology 1 @ DynaLIFE. MEDICAL EXPERT To become familiar with and apply cytological diagnostic criteria including limitations of cytology and criteria for specimen adequacy. To be able to write a concise cytology report with appropriate clinical pathologic correlations and advice re: further evaluation and/or follow-up. To be able to handle most non-gynecological and gynaecological cytologic preparations. To become familiar with fine needle aspiration techniques. To function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a case. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Manage time to maximize educational resources and opportunities. Manage workload appropriately to ensure timely completion of case work. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. 54 Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. Become fully cognizant of limitations in cytopathology to direct/advise clinicians on the further testing/management for a patient. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Review with Dr. Makarla the reporting format and protocols in place at UAH. Review specimen adequacy determination. Participate on a daily basis with case sign-out to as great an extent as possible, balancing both non-gyne and gyn cytology. Due to the breadth of non-gyn, greater emphasis is placed on non-gyn cytology at the UAH site. As this is a more senior rotation residents are expected to complete and dictate reports for review and sign-out with preceptor. Participate with cyto-histologic correlation assessment with responsible pathologist. Attend special diagnostic procedures and FNA clinic. Prepare and present Pulmonary rounds each week. Educational Materials: Required reading 1. Cibas, E.S. and Ducatman, B.S. Cytology: Diagnostic Principles and Clinical Correlates, W.B. Saunders Company, Philadelphia, 1996. 2. Orell, S.R., Sterrett, G.F., Walters, M.N-I., and Whitaker, D. Manual and Atlas of Fine Needle Aspiration Cytology, 2nd edition, Churchill Livingstone, Edinburgh, 1992. 3. Kurman, R.J. and Solomon, D. The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses. Springer-Verlag, New York, 1994. 55 Suggested supplemental reading – 1. DeMay RM. The Art and Science of Cytopathology, ASCP, Chicago, 1996. 2. Koss, L.G. Diagnostic Cytology and Its Histopathologic Bases, 4th edition, J.B. Lippincott Company, Philadelphia, 1992. 3. Bibbo, M. Comprehensive Cytopathology, 2nd edition, W.B. Saunders Company, Philadelphia, 1997 Conferences – Pulmonary Rounds. Clinical material – 1. Current cases. 2. Archival material (Please consult with cytopathologists re: personal collections and Ms Gray re: Cytotechnology Student Teaching files. 3. FNA clinic. Evaluation: Each of the weekly presentations at Pulmonary Rounds will be graded. Practical examination at end of rotation administered by Ms. Gray. Ten cases from different sites. A mark of 70% will be considered a pass mark on this examination. Overall evaluation based on standard resident evaluation form provided by Program Director’s Office. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Sept 2008 56 ROTATION SPECIFIC OBJECTIVES IN CYTOPATHOLOGY 2 @ DynaLIFE Selection: Mandatory. Site: DynaLIFE DX Diagnostic Laboratory Services Core Laboratory. Preceptor: Dr. Gordon Johnson. Length of Rotation: Two weeks Prerequisites: Cytopathology 1 & @ @ UAH, Cytopathology 1 @ DynaLIFE MEDICAL EXPERT To be able to handle most gynecological and non-gynecological cytologic preparations. To be able to write a concise cytology report with appropriate clinical pathologic correlations and advise re further evaluation and/or follow-up. Gunction effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Rstablish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage time to maximize educational resources and opportunities. Manage workload appropriately to ensure timely completion of case work. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. 57 Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: 1. Review with Dr. Johnson reporting format and protocols in place at DynaLIFE. 2. Review specimen adequacy determination. 3. Review management recommendation for gyne cytology specimens. 4. Actively participate on a daily basis with case gyne sign-out to as great an extent as possible. 5. Complete and dictate non-gyne reports for review and sign-out with preceptor. 6. Participate with cyto-histologic correlation assessment with responsible pathologist. 7. Prepare for 30-45 minute tutorial on non-gyne cytology topic with Dr. Johnson each week. 8. Prepare 30 minute CME session for presentation at biweekly cytopathology department conference. 9. Become familiar with the range of Quality Assurance activities incorporated into the practice of cytology. Educational Materials: Required reading 1. Cibas, E.S. and Ducatman, B.S. Cytology: Diagnostic Principles and Clinical Correlates, W.B. Saunders Company, Philadelphia, 1996. 2. Orell, S.R., Sterrett, G.F., Walters, M.N-I., and Whitaker, D. Manual and Atlas of Fine Needle Aspiration Cytology, 2nd edition, Churchill Livingstone, Edinburgh, 1992. 3. Kurman, R.J. and Solomon, D. The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses. Springer-Verlag, New York, 1994. Suggested reading – 58 1. Koss, L.G. Diagnostic Cytology and Its Histopathologic Bases, 4th edition, J.B. Lippincott Company, Philadelphia, 1992. 2. Bibbo, M. Comprehensive Cytopathology, 2nd edition, W.B. Saunders Company, Philadelphia, 1997 Clinical material – 2. Current cases. 2. Archival material (Please consult with Dr. Johnson). Evaluation: 1. Each of the weekly tutorials will be graded. 2. Practical examination at end of rotation. A mark of 70% will be considered a pass mark on this examination. 3. Overall evaluation based on standard resident evaluation form provided by Program Director’s office. 4. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Sept 2008 59 ROTATION SPECIFIC OBJECTIVES IN DERMATOPATHOLOGY General Pathology Residents Selection: Mandatory. Site: University of Alberta Hospital Preceptor: Dr. Ken Alanen Prerequisites: PGY2 Length of Rotation: Two weeks MEDICAL EXPERT To have a good understanding of normal cutaneous histology. To understand the stains and indications used in the diagnosis of dermatological conditions. To have a good understanding of the various techniques used to biopsy cutaneous conditions. To develop a diagnostic approach to inflammatory dermatoses. To understand the necessary information required in reporting melanoma. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). 60 Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE respond to individual patient health needs and issues as part of patient care respond to the health needs of the communities that they serve identify the determinants of health of the populations that they serve promote the health of individual patients, communities and populations acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR maintain and enhance professional activities through ongoing learning critically evaluate information and its sources, and apply this appropriately to practice decisions facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate 61 contribute to the creation, dissemination, application, and translation of new medical knowledge and practices Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL demonstrate commitment to excellence and ongoing professional development demonstrate a commitment to their patients, profession, and society through ethical practice demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation demonstrate a commitment to physician health and sustainable practice Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Residents will spend two weeks working with Dr. Victor Tron and other UAH faculty signing out dermatopathology cases. This will involve reviewing slides before sign-out, going over slides with Dr. Tron and dictating cases. Residents will also be given teaching slides to go over. Residents will also be encouraged to write up a case report. Educational Materials: Residents will be provided with teaching slide sets and text books will be recommended. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 62 ROTATION SPECIFIC OBJECTIVES DIAGNOSTIC MOLECULAR PATHOLOGY Selection: Mandatory Site: UAH Preceptor: Dr. Imran Mirza Length of Rotation: Four weeks Prerequisites: PGY-3 Specific Objectives: MEDICAL EXPERT Understand the principles, advantages and limitations of polymerase chain reaction (PCR) assays Be able to run simple PCR assays Be familiar with the common clinical applications of PCR based assays Be familiar with the latest technologies in detecting and/or quantifying PCR products, and their clinical applications Be familiar with other non-PCR based molecular techniques (such as Southern blots and fluorescence in situ hybridization) that are commonly used in molecular diagnostic laboratory Understand the issues of contamination in a molecular diagnostic laboratory, and measures taken to minimize contamination Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice Perform a complete and appropriate assessment of a patient Use preventative and therapeutic interventions effectively Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic Seek appropriate consultation from other health professionals, recognizing the limits of their expertise COMMUNICATOR Communicate effectively and demonstrate caring and respectful behavior when interacting with medical colleagues, nursing and technical staff Obtain and discuss appropriate information with staff pathologists and clinicians in difficult cases Generate written reports of excellent quality that incorporate all necessary information in a clear and concise manner Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals Accurately convey relevant information and explanations to colleagues and other professionals Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families Convey effective oral and written information about a medical encounter 63 COLLABORATOR Work as a part of a multidisciplinary team in the management and treatment of patients Ensure that reports are generated in a timely and accurate fashion for optimal patient management/treatment Participate in relevant committees Demonstrate the ability to advise on the appropriateness of obtaining samples for molecular studies Participate effectively and appropriately in an interprofessional healthcare team Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and life-long learning Demonstrate knowledge of the methods of quality control in a molecular pathology lab Participate in activities that contribute to the effectiveness of their healthcare organizations and systems Manage their practice and career effectively Allocate finite healthcare resources appropriately Serve in administration and leadership roles, as appropriate HEALTH ADVOCATE Recognize how technological advances in molecular biology may apply to improvement in diagnostic pathology Respond to individual patient health needs and issues as part of patient care Respond to the health needs of the communities that they serve Identify the determinants of health of the populations that they serve Promote the health of individual patients, communities and populations Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports SCHOLAR Develop and implement a personal continuing educational strategy Apply the principles of critical appraisal to sources of medical information Contribute to the development of new knowledge through research Participate in rounds, conferences and teaching sessions when applicableaintain and enhance professional activities through ongoing learning Critically evaluate information and its sources, and apply this appropriately to practice decisions Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion Practice medicine in an ethical manner and with a sensitivity to diverse patient populations Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner Demonstrate commitment to excellence and ongoing professional development 64 Demonstrate commitment to excellence and ongoing professional development Demonstrate a commitment to their patients, profession, and society through ethical practice Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation Demonstrate a commitment to physician health and sustainable practice Outline of Rotation: The molecular diagnostics block will take place at the Section of Molecular Pathology, the University of Alberta Hospital, over a 4-week period. An extended elective rotation with more in-depth training will be available to those are interested. The resident will learn the indications and principles of some of most widely used diagnostic molecular tests including T-cell and B-cell gene rearrangement studies for the diagnosis of lymphoproliferative disorders, detection of aberrant fusion genes in both solid and hematologic malignancies, and mutational analysis of coagulation factors. The residents will be familiar with a variety of different techniques in performing these studies, and understand their advantages and limitations. The resident will review all the results generated daily from the laboratory. He/she will obtain hand-on experience in performing some of the polymerase chain reaction (PCR) assays. The resident also will learn the principles and techniques used to prevent contamination within a diangostic molecular laboratory. The Molecular Pathology Section has been receiving an average of 200 samples per month, and it is expected that this number will grow by 20-40% over the next two years. The section is equipped with some of most up-to-dated machines including a gene sequencer and a quantitative PCR thermocycler. In addition to three full-time technologists, a full-time scientist in the Section will take up teaching responsibility. At the completion of training, the resident will have acquired the following competencies and will function effectively in the following CanMEDS roles: 1. Understand the basic principles, advantages, and limitations of various molecular diagnostic techniques commonly used in diagnostic molecular pathology laboratory Polymerase chain reaction (PCR)-based assay Southern blot hybridization In situ hybridization including FISH and CISH 2. Perform simple PCR assays and analyze data from gene sequencer and quantitative PCR thermocycler 3. Be familiar with the common clinical applications of these techniques: Diagnosis (Establishment of clonality in lymphoid cells, sub-classification of Lymphoma and leukemia, classification of soft tissue tumors, factor VLeiden, Human identity testing) Prognosis (i.e. quantitation of t(9;22)) 4. Familiar with the biology of some of the clinically important oncogenes, tumor suppressor genes and transcription factor genes lymphoma: c-myc, bcl-2, bcl-6, cyclin D1, retinoblastoma gene, p53 Precursor B-cell acute leukemia: AML1, CBF, E2A, MLL Precursor T-cell acute leukemia: tal-1, p16, p15 Acute myelogeneous leukemia: AML1, PML, MLL Chronic myelogeneous leukemia: c-abl 5. Quality control/assurance in molecular pathology 65 6. Participate in a short-term project that requires the use of some of the molecular techniques (e.g. Western blots, molecular cloning, gene transfection, FISH, gene sequencing, and genotyping) Educational Materials: 1) 2) 3) 4) 5) Educational Materials (all of these books are available in Dr. Lai’s office) Gene cloning and DNA analysis by T.A. Brown, Blackwell Publishing. 2002 PCR primer by C.W. Dieffenbach, CSHL press, 1995 Essentials of molecular biology by G.M. Malacinski, Jones and Bartlett publishers, 2003 Neoplastic hematopathology by D. Knowles, LW & W, 2002 PCR mutation detection protocols by B.Theophilus, humana press, 2002 Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 66 ROTATION SPECIFIC OBJECTIVES FOR FORENSIC PATHOLOGY General Pathology Residents Selection: Mandatory Site: Office of the Chief Medical Examiner, 7007 – 116 Street Preceptors: Chief and Assistant Chief Medical Examiner Length of Rotation: 8 weeks Prerequisites: PGY 3 MEDICAL EXPERT To familiarize the resident with the role and operation of the Medical Examiner’s Office in sudden death investigation. To teach the resident similarities and differences between hospital and forensic autopsies, including preparation of reports. To familiarize the resident with autopsy findings in a variety of sudden natural and violent deaths. To teach the resident how to properly certify cause and manner of death including knowledge of the working definitions for “cause of death”, “manner of death”, and “mechanism of death”. To introduce the resident to the external examination as an investigative procedure in Forensic Pathology. To teach the resident proper techniques for collection and handling of toxicology specimens. To teach the resident proper techniques for handling of evidence during the course of a homicide autopsy. To teach the resident the importance of photographic documentation of autopsy findings, with particular reference to homicides. To introduce the resident to the procedures used for the positive identification of human remains, with particular reference to the use of consultants in fingerprintings, odontology, radiology, and anthropology. To teach the resident the value of scene investigation in Forensic Pathology. To introduce the resident to courtroom proceedings and the presentation of evidence in court by a Pathologist. To ensure that the resident is adequately prepared for questions about Forensic Pathology on Canadian or other pathology examinations. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). 67 Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. Respond to individual patient health needs and issues as part of patient care. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. MANAGER HEALTH ADVOCATE 68 SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Tour the Medical Examiner’s facility, with the Chief or Assistant Chief Medical Examiner, and receive an introductory explanation of the methods employed by the Office to investigate sudden death. View the film, “Investigating Sudden Death: A Team Approach”. Read the Fatality Inquiries Act. Perform a minimum of 20 autopsies, under the supervision of the Chief or Assistant Chief Medical Examiner, and prepare autopsy reports in accordance with the format recommended by the preceptor. Observe a minimum of 15 external examinations, performed by the Chief or Assistant Chief Medical Examiner, and participate in the proper collection of toxicology specimens. Prepare a neuropathology report for all autopsies performed by the resident wherein the brain is kept for detailed examination. Read Petty CS: “Autopsy Records in Trauma Situations” in Curran WJ, McGarry AL and Petty CS (editors). Modern Legal Medicine, Psychiatry, and Forensic Science. FA Davis Co., Philadelphia, 1980: pp. 479-488. Read Spitz WU and Fisher RD (editors). Medicolegal Investigation of Death, Third Edition. Charles C. Thomas, Springfield, 1993. Attend all homicide scenes and homicide autopsies as an observer. Take an active role in the positive identification of tentatively identified or unidentified cases through the use of x-rays and dental records. Spend at least 1 hour with the Chief Toxicologist discussing the role of and methods used for the analysis and interpretation of postmortem toxicology pertaining to sudden death investigation. Attend a minimum of 5 non-homicidal death scenes with a Medical Investigator. Spend 1 hour with the Chief or Assistant Chief Medical Examiner learning the proper method of completing a death certificate. Attend, as a spectator, all court cases in which the Chief or Assistant Chief Medical Examiner is giving expert testimony. Evaluation: 1. On-going day-to-day evaluation. 69 2. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Sept 2008 70 THE FATALITY INQUIRIES ACT REPORTING AND INVESTIGATION OF DEATHS DEATHS THAT REQUIRE NOTIFICATION 10. (1) Any person having knowledge or reason to believe that a person has died under any of the circumstances referred to in subsection (2) or section 11, 12 or 13 shall immediately notify a medical examiner or an investigator, (2) Deaths that occur under any of the following circumstances require notification under subsection (1): (a) deaths that occur unexplainedly; (b) deaths that occur unexpectedly when the deceased was in apparent good health; (c) deaths that occur as the result of violence, accident or suicide; (d) maternal deaths that occur during or following pregnancy and that might reasonably be related to pregnancy; (e) deaths that may have occurred as the result of improper or negligent treatment by any person; (f) deaths that occur (i) during an operative procedure, (ii) within 10 days of an operative procedure, (iii) while under anesthesia, or (iv) repealed 1991 c21 s9; (v) any time after anesthesia and that may reasonably be attributed to that anesthesia; (g) deaths that are the result of poisoning; (h) deaths that occur while the deceased person was not under the care of a physician; (i) deaths that occur while the deceased person was in the custody of a peace officer or as a result of the use of force by a peace officer while on duty; (NOTE: The words "or as a result of the use of force by a peace officer while on duty" in clause (i) were added by SA 1999 c26 s9. The amending formula in that section did not specify where in the clause the words were to be added.) (j) deaths that are due to (i) any disease or ill-health contracted or incurred by the deceased, (ii) any injury sustained by the deceased, or (iii) any toxic substance introduced into the deceased, as a direct result of the deceased's employment or occupation or in the course of one or more of his former employments or occupations. INVESTIGATION OR AUTOPSY 21. The Chief Medical Examiner may at any time (a) direct a medical examiner to make an investigation into any death at any place in Alberta, or (b) authorize an autopsy of the body of any person who died under the circumstances described in section 10, 11, 12 or 13. CONDUCT OF AUTOPSY 26. (1) A medical examiner may authorize the autopsy of the body of any person who died under the circumstances described in section 10, 11, 12 or 13. 71 (2) Where a medical examiner authorizes an autopsy (a) the autopsy shall only be carried out by a pathologist; (b) the person who performs the autopsy may excise, remove and retain any part of the body or any object found in the body for the purpose of establishing the cause of death and the manner of death. REMOVAL OF TISSUE 27. Notwithstanding section 26(2)(b), a medical examiner may remove or allow the removal of tissue in accordance with the Human Tissue Gift Act, if the removal of the tissue does not interfere with any investigation or proceeding under any law in force in Alberta. RELEASE OF INFORMATION 31. (1) Except for reports, certificates and other records made in the course of a public fatality inquiry, all reports, certificates and other records made by any person under this Act are the property of the Government and shall not be released without the permission of the Chief Medical Examiner. (2) On the completion of (a) the investigation, and (b) the public fatality inquiry, if one is held, and on the receipt of a request from any of the adult next of kin or the personal representative of the deceased, the Chief Medical Examiner shall complete and send a report to the person making the request. WITNESSES 38. (1) A judge who holds an inquiry under this Act has all the powers of a commissioner appointed under the Public Inquiries Act. (2) The judge may issue or direct a clerk to issue a summons to any person who in the opinion of the judge may be able to give evidence that relates to the death under investigation. (3) Persons called as witnesses shall be examined on oath. (4) A judge has the same powers (a) to compel the attendance of witnesses, and (b) to punish a witness for (i) disobeying a summons to appear, (ii) refusing to be sworn, or (iii) refusing to give evidence, as are conferred upon a provincial judge by the Criminal Code (Canada). 72 EVIDENCE AT PUBLIC FATALITY INQUIRY 40. (1) Subject to subsection (2), a judge may admit in evidence at a public fatality inquiry, whether or not it is admissible as evidence in a judicial proceeding, (a) any oral testimony, or (b) any document or other thing, that is relevant to the purposes of the public fatality inquiry but shall refuse to admit in evidence all or part of any oral testimony or any document or other thing if he is satisfied that the oral testimony, document or other thing or part of it is vexatious, unimportant or unnecessary for the purposes of the public fatality inquiry. (1.1) Notwithstanding any other Act, regulation or other law, a judge may admit in evidence all or any relevant part of a diagnosis, record or information referred to in section 22(3) to enable him to make findings and recommendations and to report in respect of any or all of the matters set out in section 47. (2) Nothing is admissible in evidence at a public fatality inquiry that would be inadmissible in a judicial proceeding by reason of any privilege under the law of evidence. (3) If the judge is satisfied as to its authenticity, a copy of a document or other thing may be admitted in evidence at a public fatality inquiry. (4) When a document has been admitted in evidence at a public fatality inquiry, the judge may, or the person producing it or entitled to it may, with the leave of the judge, cause the document to be photocopied and the judge may (a) authorize the photocopy to be admitted in evidence in the place of the document admitted and release the document admitted, or (b) furnish to the person producing it or the person entitled to it a photocopy of the document admitted that has been certified by the judge. 73 ROTATION SPECIFIC OBJECTIVES GENERAL PATHOLOGY REVIEW Selection: Mandatory Site: - DynaLIFE DX Diagnostic Laboratory Services, Core Lab - Royal Alexandra Hospital - Misericordia Hospital - Grey Nuns Hospital Preceptor: Dr. C. O’hara/Dr. S. Brown supported by divisional directors at DynaLIFE and site leaders at the hospitals. Length of Rotation: 16 - 24 weeks. Prerequisites: Completion of PGY4 year. Specific Objectives: MEDICAL EXPERT Integrate the knowledge and training into becoming a practicing pathologist with appropriate supervision. Utilize relevant literature/resources on a case-by-case basis. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Effectively express ideas and opinions regarding the principles of pathology practice. Present current/relevant pathologic principles in a rounds format. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Share difficult/challenging cases with medical staff. Possibly participate in small research projects. Appropriately delegate when necessary. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. 74 MANAGER Demonstrate knowledge of and practice quality assurance principles. Demonstrate effective time management skills. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Communicate effectively with clinicians regarding appropriate use of laboratory tests and resources. Advocate on behalf of individual patients by ensuring appropriate and essential laboratory tests are performedRespond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Identify strengths and weaknesses, with focus on preparation for Royal College examinations. Participate in departmental rounds. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Foster good working relationships with medical, technical and support staff. Produce timely and accurate reports. Demonstrate awareness of personal limitations and address areas of weakness. Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: The resident is expected to: Review the content of the different disciplines. Identify and correct weaknesses. Function as a "practicing" pathologist with appropriate supervision. Participate in informal quizzing and more formal practice examinations at any site. Establish with the preceptor rotations in the following disciplines with approximate times: 75 - Anatomic pathology, especially cytopathology, surgical pathology and sections - four weeks. - Hematopathology - four weeks. - Clinical chemistry - four weeks. - Medical Microbiology - four weeks. - Electives and attendance at Royal College examination - eight weeks. frozen Educational Materials: Daily case material Library resources - PIP, Check Path Standard textbooks Internet resources Interesting case files Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 76 ROTATION SPECIFIC OBJECTIVES GYNECOLOGICAL PATHOLOGY Selection: Mandatory Site: Royal Alexandra Hospital Preceptor: RAH Staff Length of Rotation: Six weeks Prerequisites: PGY3 Specific Objectives: MEDICAL EXPERT Develop basic competence at examination of cervical and biopsy material. Review selected cases of routine gynecologic pathology. Achieve diagnostic competence in oncologic gynecological pathology. Achieve diagnostic competence in perinatal pathology, including: a) the ability to perform a perinatal autopsy, b) an understanding of placental pathology function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care establish and maintain clinical knowledge, skills and attitudes appropriate to their practice demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic seek appropriate consultation from other health professionals, recognizing the limits of their expertise COMMUNICATOR Formulate written reports based upon macroscopic and microscopic observations for perinatal autopsies and gynecological specimens with suitable clinicopathological correlations Assist in the continuing education of physicians and other members of the hospital staff by participating in conferences and case presentations Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals accurately convey relevant information and explanations to colleagues and other professionals develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families convey effective oral and written information about a medical encounter COLLABORATOR To demonstrate the ability to advise on the appropriateness of obtaining histological, cytological and autopsy specimens and to advise on further appropriate investigations including infection control and safety participate effectively and appropriately in an interprofessional healthcare team effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and lifelong learning participate in activities that contribute to the effectiveness of their healthcare organizations and systems manage their practice and career effectively 77 allocate finite healthcare resources appropriately serve in administration and leadership roles, as appropriate HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to gynecological processes As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health respond to individual patient health needs and issues as part of patient care respond to the health needs of the communities that they serve identify the determinants of health of the populations that they serve promote the health of individual patients, communities and populations acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports SCHOLAR Develop and implement a personal continuing educational strategy Apply the principles of critical appraisal to sources of medical information Contribute to the development of new knowledge through research Participate in rounds, conferences and teaching sessions maintain and enhance professional activities through ongoing learning critically evaluate information and its sources, and apply this appropriately to practice decisions facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate contribute to the creation, dissemination, application, and translation of new medical knowledge and practices PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion Practice medicine in an ethnical manner and with a sensitivity to diverse patient and co-worker populations Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner Demonstrate commitment to excellence and ongoing professional development demonstrate commitment to excellence and ongoing professional development demonstrate a commitment to their patients, profession, and society through ethical practice demonstrate a commitment to their patients, profession, and society through participation in professionled regulation demonstrate a commitment to physician health and sustainable practice Outline of Rotation: Cervical pathology, colposcopic biopsies, LLETZ and Cones will be available at the Royal Alexandra Hospital. A moderate amount of routine gynecologic pathology originating at the RAH will be assigned to the resident on a daily basis. A minimum of three complex gynecologic oncology cases will be assigned per week to the resident for grossing and microscopic reporting. The resident will participate in intraoperative consultations, both gynecologic and non-gynecologic, with follow-through including grossing and microscopic reporting, as arranged with the responsible pathologist, three mornings per week. The rotation should allow residents to attend 1 - 2 perinatal autopsies a week with Dr. N. Lilic. Examination of placentas in the surgical pathology division will be done on a regular basis. 78 Gynecologic consultation cases received at the Royal Alexandra Hospital will be reviewed by the resident as time permits. Educational Materials: Suggested reading – Blaustein’s Pathology of the Female Genital Tract ed. 5 (R.J. Kurman), Springer-Verlag, 2002. or, Pathology of the Female reproductive Tract ed 4 (S. Robboy), Churchill Livingstone, 2001. or, Haines and Taylor Obstetrical and Gynaecological Pathology ed. 5 (H. Fox), WB Saunders, 2002. Diagnosis of Endometrial Biopsies and Curettings: A Practical Approach ed. 2 (Robinson), Springer-Verlag, 2001. Color Atlas of Gross Placental Pathology (Cynthia Kaplan), Igaku-Shoin, 1994. The Malformed Fetus and Stillbirth: A Diagnostic Approach (R.M. Winter), John Wiley & Sons, 1988. Conferences – Multidisciplinary Gynecologic Oncology Rounds at the Cross Cancer Institute. Clinical Material – Active current cases and review of archival material. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. February 2007 79 ROTATION SPECIFIC OBJECTIVES IN HEMATOLOGICAL PATHOLOGY Selection: Site Preceptor: Length of Rotation: Mandatory. 1. University of Alberta Hospital. 2. Canadian Blood Services Dr. Gwen Clarke. Hematopathology 1A – twelve weeks. Hematopathology 1B – twelve weeks. Specific Objectives: MEDICAL EXPERT Have knowledge of peripheral blood and bone marrow morphology in health and in diseased states. Have knowledge of the basic principles underlying general hematology laboratory tests. Have knowledge of the physiology of blood clotting, the pathology of hemostatic and thrombotic disorders, and the principles of laboratory tests for the diagnosis of these disorders. Have knowledge of the techniques in blood cross matching and antibody investigation, the risks and complications associated with transfusion, the indications of blood component therapy, preparation of blood products, blood donor management and testing, and alternatives to blood products. Have an awareness of the regulations and standards that govern the field of transfusion medicine in Canada. Understand the basic principles and techniques of conventional cytogenetic analysis in hematological disorders. Function as a consultant to colleagues in appropriate laboratory test utilization and interpretation. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Communicate effectively with medical colleagues, nursing and technical staff verbally and in written reports about all aspects of laboratory hematology and transfusion medicine. Communicate effectively with patients. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. 80 COLLABORATOR Work as part of a multidisciplinary team in the management and treatment of patients. Ensure that reports are generated in a timely fashion for optimal patient management/treatment. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life. Allocate finite health care and health education resources effectively. Have in-depth knowledge of quality assurance and improvement. Understand the value of utilization reviews, transfusion audits, and continual monitoring of laboratory functioning to improve practice. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Recognize how technologic advances may apply to improvement in hematologic diagnosis, and advocate to introduce those technologies locally. Recognize alternatives to transfusion that may be appropriate recommendations to clinical colleagues and patients. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy. Apply the principles of critical appraisal to sources of medical information. Facilitate the learning of patients, students, residents and other health care professionals. Contribute to the development of new knowledge through research. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion. 81 Practice medicine in an ethical manner. Exhibit appropriate professional behavior. Be cognizant of one’s limitations of professional competence. Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Review general objectives with preceptor at beginning of Hematopathology 1A and 1B. At the beginning of each training period, the trainee will be provided with a list of his/her rotations for that twelve week period and of the specific objectives to be achieved. For a more detailed description of the outline, refer to the on-line Residents’ Training Manual at http://www.lmp.ualberta.ca/Education/Postgraduate/Anatomical_General/GP%20Manual.doc Evaluation: Written, oral and practical examination will be conducted every 6 months as part of the GP semi-annual assessment of resident performance. A mark of 70% will be considered a pass mark on this examination. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. Jan 2007 82 GENERAL OBJECTIVES HEMATOLOGICAL PATHOLOGY Introduction General Pathology residents are required to undertake twenty four weeks training in Hematological Pathology. This is divided into two twelve week blocks, the first of which generally occurs in the PGY-2 or PGY-3 year and the second in the PGY-4 or early in the PGY-5 year. Objectives are provided below for each rotation in the two blocks. These objectives are meant to serve as guidelines so that the resident is aware of the topics/assays/procedures that should be covered in each rotation. Some of them are written in a format that also allows them to be used as a checklist as a further aid to ensure that all areas are covered. The assigned preceptor for each block has the responsibility to facilitate the resident’s learning and to make sure that the resident’s knowledge is adequate by the end of the rotation. The preceptor’s signature is required at the end of each block to indicate that the curriculum for that rotation has been covered and that the resident has demonstrated the expected level of knowledge/competency. HEMATOPATHOLOGY 1A FIRST TWELVE WEEK BLOCK The first training block starts off with a fairly intensive course in Blood Banking and Transfusion Medicine, followed by a rotation through the main areas of the hematological laboratory, during which they learn basic hematological techniques and peripheral blood and bone marrow interpretation. During this rotation, the resident is also taught to perform bone marrow aspiration and biopsy. Later in the block the resident rotates through areas for specialized testing, including the special coagulation bench. BLOOD BANK AND TRANSFUSION MEDICINE The blood bank and transfusion medicine block will take place at the UAH blood transfusion laboratory over a four to six week period at the start of the residency program. The resident will gain proficiency in pretransfusion testing and will become familiar with blood components and blood component therapy, transfusion-related complications and quality assurance. Ongoing blood bank training will occur throughout the remainder of the rotation in that the resident will be the first in line to deal with blood bank problems on a daily basis (with senior staff back-up when required). 83 Methods and Procedures For each of the following methods/procedures the resident will demonstrate knowledge of the: a) principle b) test requirements including special precautions/handling c) limitations, false positives and false negatives d) interpretation e) quality control f) common alternative methods Methods/procedures:- Red cell typing Antibody screening Compatibility testing Pre warm technique Saline replacement Direct antiglobulin test Antibody investigation Elution Autoadsorption Donath Landsteiner test Drug-induced antibodies (a) (b) (c) (d) (e) (f) Blood Product/Components For each of the listed blood products or components the resident will demonstrate knowledge of: a) method of preparation b) indications for transfusion c) pretransfusion testing requirements d) volume and dosage e) potential complications f) storage requirements g) administration requirements h) special product manipulations (where applicable) i) leukodepletion ii) irradiation iii) freezing and deglycerolization iv) warming v) pooling vi) aliquoting vii) further concentrating viii) washing 84 Blood Products/components: 1. 2. 3. 4. 5. 6. 7. i) ii) iii) 8. Red blood cells Platelets Granulocytes Plasma Cryoprecipitate IVIG Other immunoglobulin fractions HBIg CMV Ig RhIg Coagulation factor concentrates including i) Factor VIII ii) Factor IX iii) Other concentrates 9. Albumin 10. Plasma anticoagulant concentrates i) Antithrombin III ii) Protein C (a) (b) (c) (d) (e) (f) (g) (h) (a) (b) (c) (d) (e) (f) (g) (h) Transfusion Practice The resident will demonstrate knowledge in the following areas of transfusion practice: I Transfusion alternatives For each of the listed alternatives the resident will demonstrate knowledge of the: a) indications b) preparation (including special manipulations) c) administration d) dose e) limitations f) contraindications g) potential complications h) efficacy/benefit i) relative cost 85 Transfusion alternatives: 1) pre operative autologous blood collection 2) perioperative hemodilution 3) perioperative cell salvage 4) pharmacologic agents i) DDAVP ii) antifibrinolytic agents iii) erythropoietin 5. pentaspan 6. hemoglobin solutions (a) (b) (c) (d) (e) (f) (g) (h) (i) (a) (b) (c) (d) (e) (f) (g) (h) (i) II Perinatal complications For each of the listed perinatal complications the resident will demonstrate knowledge of: a) presentation b) pathophysiology c) laboratory monitoring d) therapeutic interventions e) special product manipulations f) prevention Perinatal Complications: 1) Hemolytic Disease of the Newborn 2) Neonatal Alloimmune thrombocytopenia (a) (b) (c) (d) (e) (f) III Neonatal and Pediatric Transfusion Practice For each of the following aspects of pediatric transfusion practice the resident will demonstrate knowledge of the applicable: a) blood products b) special precautions/product manipulations c) laboratory testing requirements Neonatal/pediatric transfusion issues: (a) 1) CMV infection 2) Neonatal (< 4 months) transfusion 3) Exchange transfusion 86 (b) (c) IV Transfusion Complications For each of the following transfusion complications the resident will demonstrate knowledge of: a) incidence b) etiology c) diagnosis d) pathophysiology e) signs and symptoms f) time of onset (relative to transfusion) g) therapy h) prevention Transfusion Complications: 1) acute hemolytic transfusion reaction 2) delayed hemolytic transfusion reaction 3) febrile non hemolytic transfusion reaction 4) allergic transfusion reaction 5) anaphylactic transfusion reaction 6) transfusion related acute lung injury 7) transfusion-transmitted infection i) bacterial ii) HIV iii) HBV iv) HCV v) HTLV vi) CMV vii) CJD viii)Other infectious agents potentially transmitted by blood transfusion 8) Graft versus host disease 9) Post-transfusion purpura 10) Transfusion-related immunosuppression (a) (b) (c) (d) (e) (f) (g) (h) (a) (b) (c) (d) (e) (f) (g) (h) 87 V Special Transfusion Situations For each of the following special transfusion situations the resident will demonstrate knowledge of: a) blood products b) blood product dose c) special precautions Special transfusion situations: 1) 2) 3) 4) massive transfusion bone marrow transplantation solid organ transplantation chronic transfusion therapy (a) (b) (c) Dr. has demonstrated knowledge of the above topics. Preceptor for Blood Bank and Transfusion Medicine rotation: ____________________________________ Signature ________________________ Date 88 GENERAL LABORATORY HEMATOLOGY This block occurs early in the resident’s training, usually immediately following the Blood Bank and Transfusion Medicine rotation, and takes place at the University of Alberta Hospital laboratory. At the start of the rotation the resident is introduced to morphology and common hematological techniques/equipment. During this rotation, the resident will learn about hematological disorders, relevant morphological abnormalities and tests (routine and special) which may be required for their diagnosis. The basic science topics related to these areas will be addressed. The general principles of quality control and quality assurance will be taught, as well as quality control issues specific to each assay. A. General Hematology The following objectives in this section deal specifically with assays in hematological practice. Morphology and the diseases that these tests relate to are covered in subsection B entitled “Morphology”. At the end of this rotation, for each of the following assays/procedures, the Hematological Pathology resident will understand:(a) The principle of the assay (b) Indications for the assay (c) Test requirements, including special precautions/specimen handling (d) Interpretation of results (e) Limitations of the assay (including interferences, false positives and false negatives) (f) Quality control (g) Common alternative methods of performing the assay/procedure Automated complete blood count:1. Hemoglobin 2. Red cell indices (RBC, MCV, MCH, MCHC) 3. Automated white cell count 4. Automated white cell differential 5. Automated platelet count 6. Fluid cell counts/differentials (a) (c) (d) 89 (e) (f) (g) Special Tests:1. Manual white cell count 2. Manual white cell differential 3. Manual platelet count 4. Automated reticulocyte count 5. Manual reticulocyte count 6. Fluid cell counts/differentials 7. Erythrocyte sedimentation rate 8. CSF cytospins 9. Cytochemical stains 10. Erythrocyte osmotic fragility 11. Sucrose hemolysis 12. Ham test 13. G-6-PD screen 14. G-6-PD assay 15. Pyruvate kinase assay 16. Fetal cell stain 17. Hemoglobin H stain 18. Unstable hemoglobin (heat stability) 19. Unstable hemoglobin (isopropanol) 20. Hemoglobin S solubility 21. Heinz body stain 22. Plasma/whole blood viscosity (a) (b) (c) (d) (e) (f) (g) Additional topics to be addressed in this rotation include: bone marrow aspiration and biopsy - techniques, indications, risks and potential complications. The resident will be taught to perform the procedure at the start of this rotation. principles of operation of automated cell counters, standardization and quality control materials Romanowsky and other stains used in hematology slide making and slide stainers counting chambers for manual cell counts safety issues that relate to working in a hematology laboratory. These include:(a) The resident will be taught basic concepts of laboratory safety, including the use of gloves, masks, eye protection, protective garments, waste disposal, aerosol containment and use of biological safety cabinets (b) The need for universal precautions when handling blood, body fluidsand tissue will be emphasized. (c) The trainee will be taught how to clean up spills of blood and body fluids. 90 (d) Specific safety rules are available for specific areas/equipment. The resident is required to refer to the laboratory safety manual at the start of his/her rotation to take specific note of any specific guidelines for the hematology area. (As hematology is not the first rotation for the General Pathology resident, it is assumed that he/she has already been shown the laboratory safety manual and is aware of general safety guidelines. Similarly, it is assumed that the General Pathology resident is by this stage familiar with the principles of workplace hazardous material information systems (WHMIS) regulations, material safety data sheets (MSDS), fire safety, electrical safety, disposal of hazardous materials, the terms of reference of a laboratory safety committee, and accident reporting to Occupational Health and Safety and to Workers Compensation Board.) Dr. _______________________________ has demonstrated knowledge of the above topics. Preceptor for General Hematology rotation: Signature Date 91 B. Morphology (Peripheral Blood and Bone Marrow) The interpretation of peripheral blood smears and bone marrow aspiration/biopsy specimens is taught at an early stage of the residency, immediately after the resident’s basic training in transfusion medicine. During the rotation through the UAH laboratory, the trainee will be expected to participate in signing-out cases daily. The learning involves making accurate observations, interpretation of findings in a logical manner, and preparing concise and valid reports in a timely fashion. In addition to morphology recognition, the resident will learn the pathogenic mechanisms of each disorder, presentation, diagnosis, management and prognosis. At the end of the rotation, the trainee will have gained considerable experience and will be able to recognize the following: (A) the morphologic features of normal constituents in peripheral blood and bone marrow aspirates, as well as the histologic features of normal bone marrow trephine biopsy. (B) the key morphologic features of the following disease processes: I. 1. 2. 3. 4. 5. 6. 1. 2. 3. 4. 5. 6. 7. Processes involving red cells iron deficiency anemia of chronic disease anemias seen in alcoholism, chronic renal failure, endocrine disorders aplastic anemia and pure red cell aplasia thalassemia structural hemoglobin abnormalities: HbS HbC HbE others hereditary membranopathies: spherocytosis elliptocytosis stomatocytosis enzyme deficiencies: G6PD, pyruvate kinase megaloblastic anemias hemolytic anemias microangiopathic immune-mediated burns clostridium perfringes sepsis malaria hyposplenism/asplenism sideroblastic anemia / myelodysplastic syndromes congenital dyserythropoietic anemias 92 II. Processes involving white cells 1. reactive lymphocytosis 2. benign lymphoid aggregates and reactive plasmacytosis in bone marrow 3. hematogone-like cells in bone marrow 4. acute lymphoblastic leukemia 5. chronic lymphocytic leukemia 6. prolymphocytic leukemia 7. hairy cell leukemia 8. splenic lymphoma with villous lymphocytes 9. Hodgkin’s lymphoma 10. non-Hodgkin’s lymphoma low grade lymphomas intermediate grade lymphomas high grade lymphomas 11. peripheralization of lymphomas 12. large granular lymphocytosis and leukemia 13. sezary syndrome 14. adult T-cell leukemia 15. plasma cell dyscrasias including multiple myeloma, plasma cell leukemia, MGUS, amyloidosis 16. cryoglobinemia 17. toxic changes in neutrophilic granulocytes 18. myelodysplastic syndromes refractory anemia refractory anemia with ringed sideroblasts refractory anemia with excess blasts refractory anemia with excess blasts in transformation chronic myelomoncytic leukemia myelodysplastic syndromes in children 19. acute myelogeneous leukemia M0 M1 M2 M3 M4 M5 M6 M7 20. chronic myeloproliferative disorders chronic myelogeneous leukemia idiopathic myelofibrosis essential thrombocythemia polycythemia rubra vera 21. hereditary storage disorders: Gaucher disease Niemann-Pick 22. qualitative neutrophil disorders Chediak Higashi syndrome Pelger-Huet anomalies 23. mast cell disease 93 24. bone marrow involvement by histiocytosis X 25. hypereosinophilic syndrome III. Processes primarily involving platelets 1. increased peripheral platelet consumption idiopathic thrombocytopenia purpura 2. decreased platelet production megakaryocytic aplasia/hypoplasia IV. Miscellaneous 1. leukoerythroblastic anemia 2. hemophagocytic syndrome 3. granuloma 4. parasitic infestation of peripheral blood and bone marrow 5. metastatic malignancies carcinoma melanoma neuroblastoma small blue cell tumors in childhood 6. HIV infection and its hematologic complications 7. Know the role of special techniques in establishing diagnosis cytochemistry (myeloperoxidase, TRAP, sudan black, NSE, PAS) amyloid stain, reticulin stain, collagen stain commonly used immunohistochemical markers for trephine biopsy flow cytometry electron microscopy Dr. Preceptor for this rotation: Signature has demonstrated knowledge in the above topics. Date 94 C. Coagulation The resident rotates through both the routine and the special coagulation areas. During the early part of the rotation, emphasis is placed on learning the fundamentals of coagulation, understanding the disease processes that give rise to abnormal hemostasis and the screening tests for their detection. More specialized coagulation assays and indications for their utilization are covered in the latter part of the rotation, as well as in the second hematology block. Topics/disorders/disease processes to be covered in this section include:1. Basic concepts of hemostasis and thrombosis, including Role of vessel wall, platelets and humoral coagulation factors Pathways of coagulation Natural inhibitors of coagulation Platelet function Role of fibrinolytic system 3. Pathophysiology and classification of thrombocytopenic disorders 4. Diagnostic approach to bleeding disorders 5. Hereditary coagulation disorders, including treatment and complications 6. Acquired bleeding disorders, including liver disease, disseminated intravascular coagulation, renal failure, and primary fibrinolysis 7. Circulating anticoagulants, including acquired Factor VIII inhibitors in non-hemophilic patients, lupus anticoagulants and antiphospholipid antibodies 8. Congenital and acquired disorders of platelet function 9. Bleeding caused by vascular abnormalities 10. Basic principles of coagulation assays and instrumentation 11. Quality control 12. Point-of-care coagulation testing At the end of his/her coagulation training, for each of the following assays, the resident will understand:(a) The principle of the assay (b) Indications for the assay (c) Test requirements, including special precautions/specimen handling (d) Interpretation of results (e) Limitations of the assay (including interferences, false positives and false negatives) (f) Quality control (g) Common alternative methods of performing the assay 95 Assays:1. Partial thromboplastin time 2. Prothrombin time 3. Fibrinogen 4. D-Dimer (plasma) 5. D-Dimer (whole blood) 6. Thrombin time 7. Bleeding time 8. Factor XIII screen 9. Euglobulin lysis time 10. PTT inhibitor screen 11. PT inhibitor screen 12. PTT-based factor assays 13. PT-based factor assays 14. von Willebrand factor antigen 15. Ristocetin cofactor assay 16. Factor VIII inhibitor titre 17. Reptilase time 18. Platelet aggregation studies (a) (b) (c) (d) (e) (f) (g) Dr. has demonstrated knowledge of the above topics. Preceptor for Coagulation rotation: Signature Date 96 D. Quality Control and Proficiency Testing Quality control (QC) issues specific to each assay are taught at the bench, but in addition, the general principles of quality control and quality assurance are addressed during this rotation. These include:1. Basic QC principles and tools, eg. Westgard rules 2. Basic principles of quality assurance and quality improvement, including quality requirements and indicators 3. Mandate and operation of a quality council 4. Specific QC measures pertaining to each assay and piece of equipment 5. Interlaboratory benchmarking 6. Proficiency testing programs 7. Hospital and laboratory accreditation process 97 HEMATOPATHOLOGY 1B SECOND TWELVE WEEK BLOCK At the start of the second hematology block, the General Pathology resident is provided with a short refresher course through Blood Bank and the main hematology lab areas. This is required as there is usually a two-year gap between the two rotations. It also allows the resident the opportunity to go over any areas that may not have been adequately covered during the first rotation. Following the refresher course, the resident rotates through more sophisticated areas of hematology testing, such as flow cytometry, and also undertakes short rotations through the Canadian Blood Services, and the cytogenetics and HLA typing laboratories. During this second hematology block, emphasis is placed on the resident assuming greater responsibility in the laboratory, especially in handling consultations from technical staff and clinicians and in peripheral blood smear and bone marrow reporting. A. Blood Bank and Transfusion Medicine The resident undertakes a two-week refresher course in the Blood Bank, and ensures that the objectives listed for this area in the first rotation are covered. B. General Laboratory Hematology A short refresher course is provided to allow the resident to re-familiarize himself/herself in the main areas of laboratory hematology. The resident is asked to refer to the objectives listed in the first rotation for this area (including Morphology) to ensure that all topics have been covered. In addition, the following topics will be addressed:1. The principles of flow cytometry, and quality control measures in flow cytometry. 2. Applications of flow cytometry in:(a) subtyping of acute leukemias (b) T-lymphocyte subset enumeration (c) Detection of antiphospholipid antibodies C. Coagulation Coagulation topics not covered in the first hematology block are taught in this rotation. The subject material to be covered includes:1. 2. 3. 4. Hereditary hypercoagulable states, their diagnosis and management Acquired hypercoagulable states Diagnosis of thromboembolism and management Anticoagulant therapy, including prophylactic therapy, treatment of established thrombosis, monitoring of heparin and oral anticoagulant therapy, newer antithrombotic drugs, complications of anticoagulant therapy 5. Antiplatelet drugs 98 6. Thrombolytic therapy At the end of his/her coagulation training, for each of the following assays, the resident will understand:(a) The principle of the assay (b) Indications for the assay (c) Test requirements, including special precautions/specimen handling (d) Interpretation of results (e) Limitations of the assay (including interferences, false positives and false negatives) (f) Quality control (g) Common alternative methods of performing the assay Assays:1. Lupus anticoagulant detection 2. Dilute Russel’s Viper Venom time 3. Antiphospholipid antibodies 4. Antithrombin III assay 5. 6. 7. 8. Protein C assay Protein S assay APC resistance (clot-based) APC resistance (Factor V Leiden) 9. Plasminogen assay 10. Heparin-induced thrombocytopenia assay 11. Unfractionated heparin assay 12. Low mol. wt. heparin assay Dr. (a) (b) (c) (d) (e) (f) (g) (a) (b) (c) (d) (e) (f) (g) has demonstrated knowledge of the above topics. Preceptor for this rotation:- Signature Date 99 D. CANADIAN BLOOD SERVICES This short rotation through the Edmonton branch of the Canadian Blood Services familiarizes the resident with the organization of a blood transfusion service and its functions and responsibilities in the procurement, preparation and distribution of blood products. At the end of this rotation, the resident will:1. Understand the structure of the Canadian Blood Services organization, its functions and responsibilities and its relationship to health care facilities. 2. Be familiar with the management of blood donation, including (a) donor recruitment (b) donor acceptability criteria (c) confidential self-exclusion (d) collection of blood (e) potential complications of donation (f) mobile collection facilities 3. Understand the processing and testing of donated blood:(a) Automated methods for ABO and Rh testing and for detection of unexpected alloantibodies (b) Testing for transfusion-transmitted viruses (including hepatitis viruses, human immunodeficiency virus 1 and 2, HTLV-I and HTLV-II, and cytomegalovirus in selected units), and syphilis. The resident will also be familiar with confirmatory tests for positive cases. 4. Understand the methods used for preparation of blood components red cell concentrates plasma - fresh frozen plasma stored plasma apheresis plasma cryosupernatant plasma cryoprecipitate platelets (including prestorage leukodepletion) granulocytes 5. Understand the principles of apheresis donations types of apheresis machines and their principles of action applications in obtaining apheresis plasma, single donor platelets, harvesting stem cells and granulocytes care of donor, including monitoring of frequency of donation and laboratory monitoring potential complications of apheresis donation 6. Know the special requirements for transportation of blood and blood components, including different storage temperature requirements for different products. 7. Understand antenatal testing mechanism of maternal immunization serological testing of pregnant women, including antibody titres 100 8. Be aware of special manipulations required for certain products and their indications, such as irradiation of blood products, washing of red cells. 9. Understand relevant quality assurance and administration issues, eg. quality control procedures for maintaining standards in blood components quality control measures in testing for markers of infectious agents blood inventory management donor counseling Dr. has demonstrated knowledge in the above topics. Preceptor for this rotation: Signature Date 101 Hematopathology Rotation Teaching Schedule 1st rotation: 4 weeks of blood bank (2 weeks - full time; 2 weeks - half time) 2 weeks core lab 3 weeks special coagulation lab 1 week Canadian Blood Services 1 week HLA/flow cytometry 1 week vacation + longitudinal: bone marrow and peripheral blood smear morphology and special hematology 2nd rotation: 2 weeks blood bank (refresher) 2 weeks core lab 2 weeks special coagulation lab 1 week cytogenetics 1/2 week FANA's 2 weeks Royal Alexandra Hospital Laboratory 1 week vacation 1 week miscellaneous (whatever is resident's interest) + longitudinal: bone marrow and peripheral blood smear morphology and special hematology 102 E. CYTOGENETICS A two- week rotation in conventional cytogenetics will expose the resident to the basic concepts and techniques of conventional cytogenetics and its application to hematological disorders. At the end of this rotation, the resident will:1. Understand the basic principles and limitations of conventional cytogenetic studies. 2. Understand basic techniques of cytogenetic analysis. 3. Be familiar with common cytogenetic terminology. 4. Know the common cytogenetic abnormalities encountered in hematological malignancies, including those seen in acute leukemias (pediatric and adult), chronic myelogeneous leukemia and other myeloproliferative disorders, myelodysoplastic syndromes (de novo and therapyrelated cases), follicular lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, diffuse large cell lymphoma. 5. Be familiar with the specific chromosomal changes that are helpful for diagnosis of certain hematological disorders (eg. t(9;22) in chronic myelogenous leukemia) or for subtyping acute leukemias (eg. t(15;17) in acute promyelocytic leukemia). 6. Understand the prognostic significance of cytogenetic changes in certain hematological disorders, such as pediatric acute lymphoblastic leukemia, and implications for therapy. 7. Understand the utility of conventional cytogenetics in the monitoring of chronic myelogenous leukemia, with respect to (a) karyotypic evolution and impending blast crisis, (b) assessment of therapy (eg. -interferon). 8. Be aware of the use and limitations of conventional cytogenetics in minimal residual disease detection. Dr. _____________ has demonstrated knowledge in the above topics. Preceptor for cytogenetics block:- Signature Date 103 F. HLA TYPING This brief rotation is intended to provide the resident with an understanding of the role of the major histocompatibility complex (MHC), its relevance in bone marrow and solid organ transplantation and the principles of HLA typing. At the end of this rotation, the resident will:1. Understand the genetics of the MHC region and the inheritance of HLA haplotypes. 2. Be familiar with the biochemical nature of the MHC. 3. Understand the role of the MHC in normal and abnormal immunology. 4. Understand the principles of HLA typing, lymphocytotoxicity testing and mixed lymphocyte reactions. 5. Recognize the importance of HLA typing to bone marrow and solid organ transplantation. 6. Be familiar with methods of anti-HLA and anti-platelet antibody detection and with the principles of the platelet crossmatch, and recognize the utility of these assays in the selection of donors for patients who are refractory to random platelet transfusions. Dr. ___________ has demonstrated knowledge of the above topics. Preceptor for this rotation:- Signature Date 104 ROTATION SPECIFIC OBJECTIVES LYMPH NODE PATHOLOGY Selection: Mandatory Site: Cross Cancer Institute Preceptor: Dr. Raymond Lai Length of Rotation: Four weeks Prerequisites: PGY3 MEDICAL EXPERT To be familiar with the updated lymphoma classification schemes To develop a practical approach in assessing lymph node morphology To be familiar with the diagnostic criteria for major subtypes of lymphoma To be familiar with benign conditions that mimic lymphoma To have basic knowledge of using ancillary studies in making diagnosis function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care establish and maintain clinical knowledge, skills and attitudes appropriate to their practice perform a complete and appropriate assessment of a patient use preventative and therapeutic interventions effectively demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic seek appropriate consultation from other health professionals, recognizing the limits of their expertise COMMUNICATOR To communicate effectively with medical colleagues, nursing and technical staff verbally and in written reports To present cases effectively at lymphoma treatment planning meetings held weekly develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals accurately convey relevant information and explanations to colleagues and other professionals develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families convey effective oral and written information about a medical encounter COLLABORATOR To ensure that reports are generated in a timely fashion for optimal patient management and treatment participate effectively and appropriately in an interprofessional healthcare team effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict 105 MANAGER To utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life To allocate finite health care and health education resources effectively participate in activities that contribute to the effectiveness of their healthcare organizations and systems manage their practice and career effectively allocate finite healthcare resources appropriately serve in administration and leadership roles, as appropriate HEALTH ADVOCATE To recognize how technologic advances may apply to improvement in hematologic diagnosis, and advocate to introduce those technologies locally respond to individual patient health needs and issues as part of patient care respond to the health needs of the communities that they serve identify the determinants of health of the populations that they serve promote the health of individual patients, communities and populations acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports SCHOLAR To develop and implement a personal continuing educational strategy To apply the principles of critical appraisal to sources of medical information To contribute to the development of new knowledge through researchmaintain and enhance professional activities through ongoing learning critically evaluate information and its sources, and apply this appropriately to practice decisions facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate contribute to the creation, dissemination, application, and translation of new medical knowledge and practices PROFESSIONAL To deliver the highest quality of care with integrity, honesty and compassion To practice medicine in an ethnical manner emonstrate commitment to excellence and ongoing professional development demonstrate a commitment to their patients, profession, and society through ethical practice demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation demonstrate a commitment to physician health and sustainable practice Outline of Rotation: The lymph node pathology block will take place at the Cross Cancer Institute (CCI) over a 4-week period. This may be followed by a second refresher rotation in the final year, or if the resident has an interest in this area, by an extended elective rotation with more in-depth training. In addition, while on the adult clinical oncology-hematology rotation at the CCI, the resident will have the opportunity to review lymph node pathology with the pathologist at the CCI whenever time permits. 106 The resident will review all the new and consultation cases related to malignant lymphoma received during the lymph node pathology rotation. The laboratory has been receiving an average of 150 cases per month. The resident will develop a practical approach in assessing lymph node pathology, and become familiar with the use of ancillary tests (including flow cytometry, fluorescence in situ hybridization and molecular diagnostic testing) in facilitating the diagnosis of lymphoma. The resident will also experience an average of 5 bone marrow biopsies or peripheral blood smears received daily for assessment of lymphoma and myeloma involvement. An additional 5 body fluid samples will be received per week for assessing lymphoma involvement. The resident will be actively involved in ordering ancillary tests and generating final reports under the supervision of staff pathologists. Lastly, over 2000 archival lymphoma cases on file are readily available for study. At the completion of training, the resident will: Understand normal histology of various solid lymphoreticular organs, including lymph node, spleen , bone marrow and thymus. Understand the principles underlying the current WHO classification system of hematopoietic neoplasms. Know the key pathologic and clinical features of the major lymphoma entities, including Hodgkin’s lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, lymphoplasmacytic lymphoma, marginal zone lymphoma, mantle cell lymphoma, follicular lymphoma, diffuse large cell lymphoma, lymphoblastic lymphoma, Burkitt/Burkitt-like lymphoma, peripheral T-cell lymphoma Be familiar with the role of immunophenotyping by flow cytometry and immunohistochemistry in establishing a definitive diagnosis of these lymphomas. Understand the role of molecular diagnostic techniques in establishing a definitive diagnosis of lymphoma (also see “molecular diagnostics” section). Educational Materials: Suggested reading: Relevant sections of general surgical pathology textbooks (Ackerman’s, Sternberger). Jaffe ES, Harris NL, Stein H, Vardiman JW (Eds): World Health Organization Classification of Tumours. Pathology and Genetics of tumours of the Haematopoietic and Lymphoid Tissues. IARC Press: Lyon 2001. Reading material as provided by preceptor. Conferences: Lymphoma Rounds, weekly. Clinical material: Current material, including consultation cases. Archival material. Evaluation: Knowledge will be assessed on daily reviews with preceptor. Diagnostic approach, work-up and diagnostic ability assessed on an ongoing basis with current cases. 107 End of rotation practical examination. A mark of 70% will be considered a pass mark on this examination. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 108 ROTATION SPECIFIC OBJECTIVES MEDICAL BIOCHEMISTRY Selection: Site: Preceptor: Length of Rotation: Prerequisites: Mandatory University of Alberta Hospital Dr. Fiona Bamforth Medical Biochemistry 1A - 12 weeks Medical Biochemistry 1B - 12 weeks Medical Biochemistry 1A - Six months of Anatomical Pathology Medical Biochemistry 1B - Medical Biochemstry 1A, Hematopathology 1A and Medical Microbiology 1A Specific Objectives: MEDICAL EXPERT To understand the implications of age and sex-related reference intervals and other preanalytical, analytical and post-analytical factors which may affect the interpretation of tests, both physiological an artefactual. These tests include urinalysis, high volume tests, cardiac markers, enzymes, lipids, proteins, toxicological testing, trace elemental environmental toxicological testing, endocrinology, hereditary disease testing and point of care testing. To operate an adequate internal and external quality control program. To operate an adequate quality assurance program. To understand the implications of age and sex-related reference intervals and other preanalytical, analytical and post-analytical factors which may affect the interpretation of tests, both physiological and artefactual. To be able to relate the sensitivity, specificity and predictive value of tests to the individual patient. To act as consultant for clinical colleagues with respect to patient investigation and management. To draw up protocols for the appropriate investigation of patients. To develop the management skills required to appropriately equip and staff the aforementioned 300-bed hospital laboratory. To have a basic knowledge of the analytical principles involved in the instruments and techniques used in the laboratory, including proper documentation of methodologies. To develop an understanding on the use of computers and expert technology in the laboratory. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Eestablish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. 109 COMMUNICATOR Assist in the continuing education of physicians and other members of the hospital staff by participating in conferences and case presentations. Act as consultant to clinical colleagues on the interpretation and relevance of biochemical tests, with particular regard to their significance in the management of the patient Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Demonstrate the ability to advise on the interpretation of biochemical tests, to advise on the investigation of the biochemical aspects of disease in a cost-effective manner and to promote effective test utilization. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Review laboratory budgeting, workload units and other statistical approaches for assessing productivity. Review quality assurance programs available for the laboratory. Review details required for laboratory coordination of off-site testing programs, including selection of instruments and implementation of quality control and quality assurance programs for such testing. Assess details of approaches that can be used for optimizing use of laboratory tests in a costeffective manner. Review other matters pertaining to laboratory administration, including committee structures, WHMIS, accreditation, etc. Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and life-long learning. Understand the logistics of managing a hospital laboratory. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. 110 HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to biochemical disease processes. As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs. Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy. Apply the principles of critical appraisal to sources of medical information. Contribute to the development of new knowledge through research. Participate in rounds, conferences and teaching sessions. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethnical manner and with sensitivity to diverse patient and co-worker populations. Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner. Demonstrate commitment to excellence and ongoing professional development. Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: During the first rotation (twelve weeks) through the different sections of the laboratory, the associated senior staff person will ensure that experience is gained in the performance of predominantly Category I assays. During the first rotation, the resident also gains experience of sample collection procedures, separation and accessioning of specimens. During this rotation, Category II and III 111 assays will also be encountered due to clustering of tests in subspecialized areas of the laboratory and on specific instruments. The relevant senior staff persons are expected to meet with the resident on at least a weekly basis to discuss methodology, quality control and matters relevant to the ordering, patient preparation and interpretation of investigations pertinent to each area of the laboratory. The resident will also be informed of all consultation requests related to the area and be personally involved in physician/patient/laboratory interfacing. In the first month of the first rotation, the resident will spend time in the Core Laboratory and TDM/Toxicology to prepare for inclusion on the on-call rota. For the remainder of the first rotation and during the entire second rotation, the resident will be on the on-call rota as first on-call with senior staff back-up. This will allow the resident to gain experience in both consulting and problem solving in the Core Laboratory. During the second twelve month rotation, the resident will be expected to review his/her knowledge base with a shorter rotation through Core Laboratory and TDM/Toxicology. The remaining time will be spent in area of the laboratory undertaking more specialized testing and which includes more Category II and III assays. This rotation will emphasis definition of clinical problems in context of the laboratory, interpretation of results and development of consultative skills. There will be increased emphasis on quality control, quality assurance, instrument evaluation, statistics and laboratory management. During both rotations, the resident will have a weekly tutorial with the program director. In December, questions on Medical Biochemistry and a practical examination consisting of several stations are included in the in-house examination. The resident is expected to participate in bi-weekly seminar courses in Medical Biochemistry which cover the major topics in the field. Educational Materials: The Department of Laboratory Medicine and Pathology library and the John Scott library are available on-site. Residents have access to the Cameron library on campus should they require access to basic science journals. Throughout the academic year, there is a bi-weekly seminar program in Medical Biochemistry. Pathology Rounds are held weekly and the resident is expected to attend regularly and present Rounds at least once per year. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 112 GENERAL OBJECTIVES FOR MEDICAL BIOCHEMISTRY TRAINING OF GENERAL PATHOLOGY RESIDENTS The duration of training in Medical Biochemistry is twenty-four weeks. This period has been subdivided into two rotations, each of twelve weeks (Medical Biochemistry 1A and 1B). The time interval between rotations may vary for individual residents and could be as long as three years. Both rotations will be through the Division of Medical Biochemistry at the University of Alberta Hospitals and DynaLIFE core laboratories. During the first rotation through the different sections of the laboratory, the associated senior staff person will ensure that experience is gained in the performance of Category I assays. During this rotation, Category II and III assays will also be encountered due to clustering of tests in subspecialized areas of the laboratory and on specific instruments. The relevant senior staff persons are expected to meet with the resident on at least a weekly basis to discuss methodology, Quality Control and matters relevant to the ordering, patient preparation and interpretation of investigations pertinent to each area of the laboratory. The resident will also be informed of all consultation requests related to the area and be personally involved in physician/patient/laboratory interfacing. In the first four weeks of the first rotation, the resident will spend time in the Core Laboratory to prepare for inclusion on the on-call rota. For the remainder of the first rotation and during the entire second rotation, the resident will be on the on-call rota as first on-call with senior staff back-up. This will allow the resident to gain experience in both consulting and problem solving in the Core Laboratory. In the second twelve weeks of rotation, due to time constraints, the resident will be expected to review their knowledge base without formal course work. This rotation will emphasize definition of clinical problems in context of the laboratory, interpretation of results and development of consultative skills. There will be increased emphasis on Quality Control, Quality Assurance, instrument evaluation, statistics and laboratory management. During both rotations, the resident will have a weekly tutorial with the program director. At the end of each twelve week rotation there is an examination. The resident is expected to participate in weekly seminar courses in Medical Biochemistry. There are two one-year courses run consecutively and participants present a 45 minute lecture followed by discussion. 113 A. SPECIMEN COLLECTION/ACCESSIONING Patient preparation, types of blood containers, anti-coagulants, collection techniques, universal precautions. Understand preanalytical variables and their effect on test results. Specimen separation, patient demographics, accessioning, specimen tracking, sample handling and storage, referral of samples to other laboratories. B. URINALYSIS urinalysis test for glucose, protein, pH, bile, blood, ketones, specific gravity, urobilinogen, leucocytes. Correlate abnormals with microscopic exam microscopic exam cells, casts, crystals reducing substances causes of positive tests stool analysis occult blood, fat, trypsin, microscopy renal function tests osmolality, water deprivation tests, ammonia, titratable acid Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Read protocols for testing where appropriate. 3. Examine and document a minimum of 40 urine sediments. 4. Become familiar with as many abnormal urine constituents as possible. 5. Be aware of progressive testing protocols if relevant. C. HIGH VOLUME TESTS General tests Include: sodium, potassium, chloride plasma, urine, sweat, and other body fluids total CO2 Correlation with pH, pCO2, anion gap urea, creatinine Serum and urine. Clearance calculations. Interpretation in renal disease 114 glucose Current diagnostic criteria for diabetes mellitus and gestational diabetes. Interpretation of glucose tolerance tests. Physiological variations. total protein, albumin Standardization. Physical properties of proteins. Deproteinization techniques. calcium, magnesium, phosphate, urate Variations in bone and renal disease bilrubin Interpretation in different disease Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Be aware of progressive testing protocols if relevant. 3. Observe start up procedure, calibration procedure and quality control of appropriate high throughput analyzers. 4. Compare mean, SD and CV of controls on the high throughput analyzers for electrolytes, creatinine, urea and glucose from month-to-month. 5. Calculate mean and SD of the anion gap in 40-50 patients. 6. Calculate five creatinine clearances ± correction for surface area with normal and abnormal serum creatinine. Understand different formulae for calculating creatinine clearance and their limitations. 7. Compare calculated vs measured osmolalities in 20 patients with normal and abnormal. Calculate mean and SD of the difference between them. 8. Tabulate data for calculated vs measured osmolar gaps on patients with alcohol excess. 9. Understand logistics of high volume analyzers (tests per hour, accommodation of stat samples). 10. Understand relative costs - reagents, disposables, services, maintenance contracts, technologist time, capital cost. Cardiac Markers Include: CK, CKMB, troponin I Time course variations in myocardial infarction. Sensitivity and specificity for myocardial infarction. Enzymes 115 ALT, AST, GGT, alkaline phosphatase interpretation in liver disease Lipase, amylase Diagnosis of pancreatitis limitations of each test and Lipids total cholesterol, triglycerides, HDL and LDL cholesterol, apo A1, apo B100 screening, current guidelines for reporting cardiac risk, calculation of derived parameters Miscellaneous tests ferritin, serum iron, TIBC, vitamin B12 folate, homocysteine Biochemical parameters in diagnosis of hematological disorders. Correlation with hematologic findings. cholinesterase total activity and dibucaine number myoglobin criteria for analysis microalbumin Rationale for testing and monitoring diabetes mellitus HbA1C Interpretations of results, interference from other hemoglobins Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Observe start up procedure, calibration procedure and quality control of appropriate analyzers 3. Understand rationale for testing for each analyte and be aware of progressive testing protocols. D. TDM/TOXICOLOGY Quantitative drug assays automated technologies: e.g. phenytoin, acetaminophen, methotrexate, quinidine chromatography instrumentation: quantitative assays by manual methods e.g. clonazepam, clozapine, imipramine, alcohols, cyclosporin, tacrolimus, sirolimus e.g. OKT3 (ELISA), 116 barbiturates spectrophotometry (UV Other quantitative assays quantitative assays by chromatography instrumentation e.g. urine metanephrines, catecholamines Qualitative toxicology assays Qualitative toxicology assays by immunoassay, thin layer chromatography, GC-MS. Include: 1. Appropriate specimen collection protocols, including reference to pertinent pharmacokinetic principle. 2. Sample pretreatment if relevant 3. Be aware of specimen adulteration and substitution issues. 4. Review/interpret/clarify information accompanying TDM requests (communication with requesting physicians, requisition review, gain experience in appending interpretive comment). 5. Be aware of pharmacokinetic factors influencing levels. 6. Be aware of method performance issues including sensitivity, specificity, imprecision etc. 7. Be aware of progressive testing protocols, protocols, treatment and laboratory implications (e.g. dialysis with methanol hemodialysis prediction). Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Understand principles of: a) quantitative drug assays on automated high throughput immunoassay analyzers e.g.: enzyme multiplied immunoassay technique cloned enzyme donor immunoassay fluoroescence polarization immunoassay microparticle enzyme immunoassay b) Gas chromatography with nitrogen, phosphorus, electron capture and flame ionization detection c) head space gas chromatography 117 d) high performance liquid chromatography e) gas chromatography-mass spectrometry f) LC/MS/MS g) Toxilab TLC h) manual assays - ELISA, UV spectrophotometry E. PROTEINS Include: serum protein electrophoresis interpretation of patterns, monoclonal bands CSF protein electrophoresis interpretation of oligoclonal bands urine protein interpretation of patterns, monoclonal bands, Bence-Jones proteins Hemoglobin electrophoresis using electrophoresis at different pH identification and significance of variants hemoglobin A2 quantitation and significance specific proteins (e.g. transferrin, significance in disease ceruloplasmin, alpha-1-antitrypsin) Immunoglobulin quantitation, Cd, C4, CH50, C1 esterase inhibitor significance in disease immunofixation interpretation and significance IgE, RAST testing testing interpretation and limitations of serum tumour markers (PSA, CEA, hCG, AFP) limitations and uses of tumour markers patterns, identification F. MISCELLANEOUS TESTS Include: gastrointestinal function tests: carotene, vitamins, fecal fat, fecal and serum bile acids significance/limitations in assessing gut function renal function: 118 of oxalate, citrate, cystine, calculus analysis significance of results porphyrins, PBG, ALA screening and quantitation methemoglobin, sulfhemoglobin Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Understand principles of: a) high performance liquid chromatography b) manual/semi-automated enzyme kinetics c) fluorometric analysis d) spectrophotometric G. ENDOCRINOLOGY Include: thyroid function tests (TSH, FT4, T3, TBG, T3 uptake) diagnosis of thyroid disease, progressive testing protocols cortisol, ACTH diagnosis of adrenal disease estrogens, androgens, FSH, LH tests of gonadal/pituitary function insulin, C-peptide, GH, glucagon disorders of growth and glucose regulation hCG diagnosis and monitoring of pregnancy calcitonin, PTH, vitamin D test of calcium/bone function aldosterone, renin role in hypertension Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Understand principles of: 119 a) automated and semi-automated immunoassay analyzers b) scintillation counters 3. Understand principles and interpretation of stimulation and suppression tests. H. TRACE ELEMENTS/ENVIRONMENTAL TOXICOLOGY Include: Ca, Mg, Cu, Zn, Fe, Li serum urine and other fluid levels. Deficiency and therapeutic/toxic levels Pb, Hg, A1, As toxic levels, clinical effects and treatment Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Understand principles of atominc absorption spectroscopy (flame, graphite furnace, cold vapor). 3. Check clinical details of any abnormal results. I. HEREDITARY DISEASE Include: neonatal screen on blood spots: TSH (± T4) amino acid chromatography biotinidase organization of provincial screening programs. population statistics. metabolic screens: sugar identification urine/serum amino acids, mucopolysaccharides, oligosaccharides, organic acid analysis limitations of metabolic screens amino acid quantitation use in diagnosis and monitoring treatment metabolic testing: carnitine lysosomal enzymes Gal-1-PUT role in diagnosis and monitoring of inherited metabolic disorders maternal serum prenatal screen: principles 120 of multivariate analysis, AFP, UE3, hCG limitates in testing for NTD and Down syndrome Procedures 1. Read method manual for each test and understand principle of each test, sensitivity, specificity, linearity, interferences etc. which may affect the patient results. 2. Understand principles of population screening for disease. 3. Understand principles of: a) thin layer chromatography b) gas chromatography-mass spectrometry for organic acid analysis c) high performance liquid chromatography for amino acids, including total homocysteine d) spectrophotometry e) fluorometry J. POINT OF CARE TESTING Procedures 1. Become familiar with the advantages and limitations of point-of-care (POC) testing. 2. Know how to compare the costs of POC testing to central laboratory testing. 4. Know how to evaluate and introduce POC testing into a hospital or ambulatory care environment. Check list: A. Specimen collection/accessioning B. Urinalysis Analytes ٱ Procedures ٱ General Tests Analytes ٱ Procedures ٱ Cardiac markers Analytes ٱ Procedures ٱ Enzymes Analytes ٱ Procedures ٱ C. High Volume Tests 121 Lipids Analytes ٱ Procedures ٱ Miscellaneous tests Analytes ٱ Procedures ٱ Quantitative drug assays Analytes ٱ Procedures ٱ Other quantitative assays Analytes ٱ Procedures ٱ Qualitative Toxicology Analytes ٱ Procedures ٱ E. Proteins Analytes ٱ Procedures ٱ F. Miscellaneous tests Analytes ٱ Procedures ٱ G. Endocrinology Analytes ٱ Procedures ٱ H. Trace elements/ environmental toxicology Analytes ٱ Procedures ٱ I. Hereditary disease Analytes ٱ Procedures J. Laboratory management Procedures ٱ K. Point of care testing Procedures ٱ D. TDM/Toxicology 122 Medical Biochemistry Rotation Schedule Rotation 1 Week 1 Staff contact George Cembrowski 2 3 4 5 6 7 8 9 10 11 12 Area Specimen handling, accessioning, urinalysis TDM/toxicology TDM/toxicology Core Laboratory Core Laboratory Hereditary diseases Point of care/QA Special investigations DynaLIFE DynaLIFE DynaLIFE DynaLIFE Rotation 2 Week 1 2 3 4 5 6 7 8 9 10 11 12 Area TDM/Toxicology QA/lab management Core Laboratory Molecular genetics I Molecular genetics II Hereditary diseases Endocrinology Special investigations DynaLIFE DynaLIFE DynaLIFE DynaLIFE Staff contact Don LeGatt George Cembrowski George Cembrowski Martin Somerville Raymond Lai Fiona Bamforth Connie Prosser Connie Prosser Trefor Higgins Trefor Higgins Trefor Higgins Trefor Higgins Don LeGatt Don LeGatt George Cembrowski George Cembrowski Fiona Bamforth George Cembrowski Connie Prosser Trefor Higgins Trefor Higgins Trefor Higgins Trefor Higgins 123 ROTATION SPECIFIC OBJECTIVES MEDICAL MICROBIOLOGY Selection: Site: Preceptor: Length of Rotation: Prerequisites: Mandatory University of Alberta Hospital Dr. Kinga Kowalewska Medical Microbiology 1A – 12 weeks Medical Microbiology 1B – 12 weeks Microbiology 1A – Six months of Anatomical Pathology (minimum) Microbiology 1B – Microbiology 1A, plus Haematopathology 1A, Plus Medical Biochemistry 1B, plus, at least, fifteen months of Anatomical Pathology (minimum) Specific Objectives: MEDICAL EXPERT To become familiar with testing and reporting practices in medical microbiology in acute care and community based medical microbiology To interact with medical colleagues to assist in making decisions about correct microbiological diagnoses To understand how microorganisms respond to various therapies and to assist other physicians in selection of the most appropriate treatments. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Assist in the continuing education of physicians and other members of the hospital staff by participating in conferences and case presentations Act as consultant to clinical colleagues on the interpretation and relevance of microbiological findings, with particular regard to their significance in the management of the patient Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. 124 COLLABORATOR Demonstrate the ability to advise on the appropriateness of obtaining microbiological specimens and to advise on further appropriate investigations Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and life-long learning Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to medical microbiological processes As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy Apply the principles of critical appraisal to sources of medical information Contribute to the development of new knowledge through research Participate in rounds, conferences and teaching sessions Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. 125 PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion Practice medicine in an ethnical manner and with a sensitivity to diverse patient and co-worker populations Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner Demonstrate commitment to excellence and ongoing professional development Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Microbiology 1A. The resident will develop an understanding of appropriate specimens, transport conditions and laboratory techniques for optimal recovery of microorganisms causing infections. Most of this time will be spent in bacteriology The resident will learn the differences between normal flora and the conditions where potential pathogens cause infections. The resident will develop an understanding of laboratory bench protocols by observation and hands on experience so that they become good communicators with their clinical colleagues. The resident will learn the mechanisms of antimicrobial action and the most appropriate methods for identification of antimicrobial resistance. The resident will learn the units of medical microbiology and how they are incorporated into the laboratory report. The resident will participate in rounds conferences and seminars on various topics of medical microbiology The resident will take graded call responsibilities in the last 7-8 weeks with immediate staff person backup in order to develop the skills necessary to interact with other clinicians. Microbiology 1B The resident will undertake further rotations in medical microbiology to broader the base of understanding in all aspects. These rotations include, viral culture and serology, parasitology, mycology, molecular diagnostics (identification of specific pathogens, strain typing, sexually transmitted diseases identification, etc). The resident will take part in infection prevention and control activities The resident will develop an understanding of laboratory management tools by participating in senior microbiology meetings. The resident will participate in community based microbiological investigations to develop an understanding of differences between tertiary care, general hospital, and community microbiology. The resident will participate in regular conferences, rounds and seminars The resident will have an opportunity to engage in short term research activities to develop investigative skills. 126 The resident will participate actively in on call service and act as a senior resident in these duties, with back up by a staff person. Educational Materials: These materials are available to the residents in the Resident’s Room, Staff Personnel offices or the main diagnostic laboratories. Texts: Manual of Clinical Microbiology , 8th Edition ASM Press Color Atlas and Textbook of Diagnostic Microbiology. Koneman et al. Lippincott Principles and Practice of Infectious Diseases. Mandell et al. Churchill Livingstone Manuals: Clinical Microbiology Procedures Handbook. Isenberg. ASM Press. University of Alberta Hospital, Laboratory Procedures Manual Dynacare Kasper Medical Laboratories Procedures Manual Journals: (available in the Microbiology and Public Health Library) Journal of Clinical Microbiology. ASM Antimicrobial Agents and Chemotherapy. ASM Diagnostic Microbiology and Infectious Diseases. Elsevier Journal of Clinical Virology. ASM Medical Mycology and Mycoses. Other journals are available in the John Scott Library, University of Alberta Computer: Residents have a computer available to them in the residents’ room. Access to PubMed and other sites is available on-line. Evaluation: Regular topic sessions are held with the preceptor to evaluate progress. End of rotation examinations (held in conjunction with General Pathology or with Medical Microbiology residents) Final examinations at the end of rotation 1B, including written, practical and oral exams. Evaluations of co-workers (other staff persons, technologists, and clinicians) and of the preceptor. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. May 2007 127 GENERAL OBJECTIVES MEDICAL MICROBIOLOGY INTRODUCTION Regardless of the size and degree of sophistication of the laboratory, there are five basic steps involved in the process of microbial identification and antibiotic susceptibility testing. These are: collection of the specimen to be processed initial processing of the specimen for culture identification of potentially pathogenic bacteria in the specimen antibiotic susceptibility testing of selected medically important bacteria, and final report of results to the physician The purpose of this handout is to describe the various ways specimens are collected for the Diagnostic Microbiology Laboratory. SPECIMEN COLLECTION BASIC CONCEPTS 1. The clinical specimen must be material from the actual infection site and must be collected with a minimum of contamination from adjacent tissues, organs, or secretions. 2. Optimal times for specimen collection must be established for the best chance of recovery of causative micro-organisms. 3. A sufficient quantity of specimen must be obtained to perform the culture techniques requested. 4. Appropriate collection devices and specimen containers must be used to ensure optimal recovery of microorganisms. 5. Whenever possible, obtain cultures prior to the administration of antibiotics. 6. The culture container must be properly labelled: name hospital number (PHN number) source physician date/hour 128 A. SPECIMENS FROM DIFFERENT BODY SITES 1. Specimens from Body Sites that Harbor a Normal Flora A specimen obtained from a body site that harbors normal flora will always have components of the flora in it. Thus, no attempt is made to prevent contamination of these specimens. Since components of the normal flora are expected to be in these specimens, they will be examined only for the presence of unexpected organisms that are not members of the normal flora. Table 1 lists those sites that harbor normal flora. 2. Specimens from Normally Sterile Body Sites Located Near an Area that Harbors Normal Flora For certain body sites that do not harbor normal flora, specimens are collected from secretions or excretions that must pass through a second site that does harbor normal flora. Such specimens will often contain components of the flora of the second site. However, certain steps can be taken to minimize contamination of these specimens so that the normal flora will not interfere with recovery of the etiologic agent. For example, the kidney and bladder are sterile in health. However, urine is voided through the external opening of the genitourinary tract which harbors normal flora. Thus, to minimize contamination of the urine, the external genitalia are cleaned with soap and water and the first several milliliters of urine are discarded before the urine is collected for culture. Examples of body sites that are normally sterile but secretions/excretions from them must pass through a second site with normal flora are listed in Table 1. 3. Specimens from Normally Sterile Body Sites that do not Produce Secretions or Excretions There are certain body sites that do not harbor normal flora and do not produce secretions or excretions (Table 1). Specimens from such sites are collected by aseptic invasive procedures and thus the presence of any microorganism is indicative of disease. All precautions must be taken to avoid any contamination of these specimens. Since many of these invasive procedures involve insertion of a sterile needle through the skin which harbors normal flora, the skin is cleansed with alcohol and an iodine solution to prevent contamination of the needles. Examples of body sites that fall into this third category are listed in Table 1. 129 TABLE 1 Goals in Prevention of Specimen Contamination with Normal Flora Based upon the Body Site Infected I. Body sites that harbor a normal flora. No attempt is made to reduce contamination of the specimen with the flora. Examples: Skin Conjunctiva Upper respiratory tract (nose, throat) Mouth Lower gastrointestinal tract External genitalia Anterior urethra Vagina/Endocervix II. Body sites that are normally sterile but produce secretions/excretions that pass through a second site harboring a normal flora. Attempts are made to reduce contamination of secretion/excretion by normal flora from second site. Examples: Urinary tract (bladder, ureter, kidney) Salivary glands Lower respiratory tract Sinuses III. Body sites that are normally sterile and do not produce secretions/excretions. Any contamination of the specimen must be prevented. Examples: Blood, Brain, Joint, Bone 130 B. SPECIMEN COLLECTION TO MAXIMIZE RECOVERY OF THE ETIOLOGIC AGENT Certain factors influence the recoverability of etiologic agents of infection. These are: 1. the material used to collect the specimen, the environment in which the specimen is placed, and... the time delay between obtaining the specimen and transporting it to the laboratory Material Used to Collect the Specimen Certain types of swabs and fluids that are used to collect specimens may contain substances that are inhibitory to various pathogenic bacteria. Thus, in certain instances, the type of material used to collect the specimen must be selected on the basis of the pathogen suspected to be present. For example, in cases of suspected gonorrhea, special calcium alginate swabs must be used to collect the specimen. Cotton swabs should not be used because they are inhibitory to the pathogen responsible for this disease. 2. Environment of the Specimen Once the specimen is obtained, it must be placed in an environment that will maintain the viability of the pathogen. Some pathogens are very sensitive to drying and thus specimens should always be kept in a moist environment. The use of special transport media often provide adequate moisture if the specimen is on a dry swab. Also, some pathogens are extremely sensitive to oxygen. Specimens suspected of containing these pathogens (obligate anaerobes) must be kept in an oxygen free environment. The use of an oxygen-free syringe to collect the sample or a variety of commercially available anaerobic containers will provide an adequate environment for these oxygen sensitive organisms. 3. C. Avoid Time Delays INITIAL PROCESSING OF SPECIMENS FOR CULTURE Some specimens are examined immediately upon receipt in the laboratory. examination is either for the purpose of: 1. This direct determining the probable identity of the etiologic agent, or ... assessing the acceptability of the sample for culture. Probable Identity of the Etiologic Agent Certain specimens are examined microscopically when they are received in the laboratory in an attempt to rapidly identify the etiologic agent of infection. Most of these examinations are performed on specimens that have been stained, although some are performed on unstained preparations. Most of the tests provided on presumptive identification of the etiologic agent and must be confirmed by further tests. In general, direct examination of a specimen can yield useful information about the etiologic agent of disease if the specimen: - does not contain contaminating normal flora (thus the presence of any bacteria indicates infection), or... 131 - contains a pathogen with a distinct morphology and/or staining reaction that allows it to be recognized even in the presence of contaminating normal flora. 2. Acceptability of Specimen for Culture Many laboratories have begun to perform Gram stains on all sputum specimens they receive for culture. This is to determine if the specimen is truly a good sputum (that has been expectorated from the lower respiratory tract) and not merely saliva from the upper respiratory tract. If, on Gram stain, the specimen is found to contain numerous inflammatory cells with few or no epithelial cells, this indicates that the specimen is sputum and therefore acceptable for culture. If, on the other hand, the specimen contains few inflammatory cells and many epithelial cells (sloughed from the mucous membranes of the upper respiratory tract), it is not acceptable for culture, and another specimen must be obtained from the patient. This process of screening sputums for acceptability prevents expensive cultures from being performed on specimens that are inappropriately obtained and would not be likely to yield the etiologic agent. D. IDENTIFICATION OF MEDICALLY IMPORTANT BACTERIA Initial Examination of Primary Cultures After overnight incubation of the primary cultures, they are examined for bacterial growth that is indicative of the presence of infection. As mentioned previously, the mere presence of bacteria on the cultures is not always indicative of infection. The precise interpretation of the cultures varies with the type of specimen involved. 1. Cultures of Specimens that Should be Sterile in the Absence of Infection The presence of any bacteria in cultures inoculated with specimens obtained by an aseptic procedure from a body site that is normally sterile is indicative of infection. Examples of specimens that would fall into this category are listed in Table 2. The only situation in which infection is not indicated is if there is some reason to suspect the specimen was inadvertently contaminated when it was obtained. 2. Cultures of Specimens that are Contaminated with Normal Flora As mentioned previously, specimens from body sites that either normally harbor flora or are near another site that harbors flora are usually contaminated to some degree (Table 2). In some instances, selective media have been used to inhibit the normal flora and cultures are more easy to interpret. However, in many instances, selective media cannot be used. Many agents that inhibit the flora also inhibit the pathogens that might be present. Thus, cultures of these specimens must be examined for the presence of bacteria that are not components of the normal flora. Since the bacteria that comprise the normal flora vary widely with each body site, what is normal flora for one site may be a pathogen for another site. Therefore, the interpretation of what is normal and what is potentially pathogenic, changes with the type of specimen involved. Those bacteria considered to be potentially pathogenic are subjected to further tests. 132 TABLE 2 General Guidelines for the Initial Examination of Primary Cultures Specimens Expected to be Sterile in the Absence of Infection Specimens Expected to be Contaminated with Normal Flora blood urine spinal fluid sputum joint fluid swabs of infected site ** tissue biopsy* drainage from infected site** aspirate from infected tissue* feces * if body site normally sterile ** if body site normally harbors a flora or is near a site that harbors a flora 133 E. LABELING AND REJECTION OF SPECIMENS 1. Requisitions It is important that the laboratory requisitions include enough information to enable the laboratory to do the best job. All that the laboratory knows about the patient is learned from the requisition. All microbiology requisitions should include information about the source, the diagnosis and/or history, and the test requested. Source The source should be specific. It is not enough to list wound or drainage. The source should include the anatomic location and type of wound. Is it from the head or the leg? Is it a bite, a puncture wound, a surgical incision or a skin abrasion? Diagnosis/History The diagnosis can sometimes help the technologist suspect growth of a specific organism that may require special media, a different incubation environment, or a longer incubation time. Additionally, knowing that the patient has osteomyelitis or pyelonephritis can affect the extent to which an organism is characterized and can determine the need for antimicrobial susceptibility testing. Knowledge of a patient’s antibiotic therapy is useful for correlation of test results. Test Requested Every test requested should have a written order on the patient’s chart. 2. Unacceptable Specimens The information on the requisition must match the information on the specimen label. If names or sources do not match, the specimen should be recollected. The laboratory should not assume the responsibility of liability of questionable information. Perhaps the best approach to this issue is to divide specimens into two groups - invasive and noninvasive. A noninvasive specimen, such as urine, sputum, stool, and wounds, must always be recollected if it is received unlabeled or there is a misidentification. An invasive specimen, such as blood culture, sterile body fluid, amniotic fluid or an operating room specimen, may be processed if the person responsible for the error comes to the laboratory and signs a release form. This places all responsibility and liability on the person signing the form. All rejected specimens have written documentation sent to the collection site to verify receipt of the specimen in the laboratory and the reason for its rejection. 134 Other suboptimal specimens that are rejected include the following: leaking specimens syringes with needles attached stools contaminated with urine or barium anaerobes on inappropriate sources unpreserved specimens older than 2 hours refrigerated blood cultures dried up specimens specimens in formalin Processing a suboptimal specimen will always yield suboptimal results. TABLE 3 Direct Examination of Specimens for Identification of Etiologic Agents Suspected Infection Meningitis Specimen Test Etiologic Agent Spinal fluid Gram stain Any bacteria seen Syphilis Scraping from chancre Darkfield Spirochetes (Treponema pallidum) Gonorrhea Urethral exudate (males only) Gram stain Gram-negative diplococci (Neisseria gonorrhoeae) Septic arthritis Joint fluid Gram stain Any bacteria seen Abscess Aspirate from abscess Gram stain Any bacteria seen Anaerobic soft tissue Aspirate from infection Gram stain Tuberculosis Sputum Acid-fast stain Predominant bacteria possessing bizarre morphology characteristic of certain anaerobes Acid-fast bacilli (Mycobacterium tuberculosis) Pneumocystis pneumonia Sputum/deep respiratory secretions Gomori’s methenamin e-silver stain 135 (Pneumocystis carinii) F. INOCULATION OF PRIMARY CULTURES Once a specimen has been received by the laboratory and appropriate direct examination has been performed, it is inoculated onto a series of media for culture. These initial cultures are called primary cultures. 1. Selection of Media for Primary Cultures Two major factors determine the media to be inoculated for primary cultures. They are the source of the specimen and the organisms most likely to be in the specimen. Certain types of organisms cause infections at particular sites more frequently than at other sites. Media for the recovery of organisms unlikely to be present are not routinely inoculated unless specifically requested by the physician. Finally, those specimens, like blood and spinal fluid that usually contain only a single type of pathogen in low numbers, are inoculated into liquid media to ensure recovery of the pathogen. Once the media for the primary cultures have been selected, they are inoculated with a small portion of the specimen. As mentioned previously, if the specimen is urine, cultures must be inoculated in such a fashion as to allow counting of the number of bacteria present as well as their recovery. After the appropriate media have been inoculated, the cultures are incubated overnight at 37˚C (normal human body temperature) either in a normal air atmosphere (media for nonfastidious Gram-negative organisms), at atmosphere with increased C0 2 (media for Gram-positive and fastidious Gram-negative organisms), or an anaerobic atmosphere (media for obligate anaerobes). 2. Identification of Pathogenic Organisms From the initial examination of the primary cultures, potentially pathogenic organisms are selected for further identification tests. The degree to which an organism is identified varies among laboratories and is determined to some extent by the organism itself. G. ANTIBIOTIC SUSCEPTIBILITY TESTS Pathogenic bacteria recovered on primary cultures are tested for their susceptibility to a variety of antibiotics. These tests are usually performed at the same time as identification tests. The results of the tests are ultimately used by the physician as a guide for selection of antimicrobial therapy for the patient. Susceptibility tests are not performed on bacteria that are predictably susceptible to antimicrobial agents commonly used to treat infections caused by these bacteria (e.g. Streptococcus pyogenes is universally susceptible to penicillin). In addition to the unpredictable susceptibility profile of a potential pathogen, other factors must be considered when determining whether antimicrobial susceptibility is warranted: body site - susceptibility tests are not performed on bacteria that are isolated from the anatomic site of which they are normal inhabitants (i.e., do not test E. coli isolated from stool but would if E. coli isolated from blood). 136 presence of other bacteria and quality of specimen - e.g. colony count is important in urine. host status - some bacteria, which may be normal, may cause disease in an immunocompromised patient. A primary objective of antimicrobial therapy is to use the least toxic, most cost-effective, and most clinically appropriate agents and to refrain from more costly, broad-spectrum agents when they are unnecessary. There are certain guidelines that are used when reporting antimicrobials. As a general guideline, it is suggested that with a particular antimicrobial class, primary (group A) agents be reported first, and that secondary (group B) agents are reported only if one of the following conditions exists: the isolate is resistant to the primary agents the patient cannot tolerate the primary agents the infection has not responded to the primary agents OR a secondary agent would be a better clinical choice for the particular infection. For example, a primary cephalosporin, such as cephalothin or cefazolin (first generation cephalosporin), would be a reasonable choice for a susceptible ≥ - 2nd generation (cefoxitin) or 3rd generation (cefoxaxime) would not be required. An exception would occur in meningitis, because only 3rd generation cephalosporins effectively cross the blood-brain barrier. H. Role of Normal Flora In the diagnostic approach to all types of infections, one must consider the types or organisms normally found at the site to be able to determine whether diagnostic microbiology data indicate the presence of a pathogen. This requires a knowledge of the normal flora to be expected at each site, the clinical setting and the patient’s presentation. 1. Discriminating Between Normal Flora and Pathogenic Microorganisms The consensus concerning what is considered “normal flora” can change with time as new associations between organisms and disease states are recognized. 2. The Immune Status of the Host The defenses of the host with a suspected infection must be evaluated when determining whether the identification of a specific microorganism is likely to be significant in causing disease. When considering the likelihood that a microorganism will cause infection in a given host, both the virulence of the organism and the defenses available to the host to counteract the establishment and progression of infection must be considered. At one end of the spectrum of organisms are the normal flora that usually exist as “commensals” for the life of the normal host without causing disease. At the other end are organisms that often establish infection and cause disease in the normal host when present in numbers above a certain threshold. For our purposes, a normal host is considered to be one with mature immunologic defenses but without specific immunity against the microorganism in question. In an immunocompromised host, however, microorganisms that are usually not pathogens in a normal host may cause serious infection. This type of infection is referred to as an “opportunistic” infection to indicate that a combination of a reduced host response and a 137 pathogen of low virulence has resulted in the establishment of infection. Of course, organisms exhibiting high levels of virulence also cause disease in immunocompromised hosts. Since the host response is diminished, infections with virulent pathogens are usually more severe and more rapidly progressive than in the normal host. 3. History Travel and food ingestion history are particularly important in gastrointestinal infections and food poisoning. A history of travel to countries with less effective sewage sanitation facilities greatly increases the risk of acquiring an enteric infectious pathogen. I. Laboratory Diagnosis of Viral Infections The cardinal rules in specimen collection are as follows: 1. Proper Time Obtain the specimen early in the disease process. 2. Appropriate Means of Transport Keep the specimen cool and moist (i.e. refrigerated or on ice). 3. Proper Specimens Go “where the action is”. Methods in Diagnostic Virology There are three major ways for the laboratory to diagnose viral infections: 1. 2. Direct Detection of the Virus in the Clinical Specimens immunofluorescence staining (IF) enzyme linked immunosorbet assay (ELISA) electron microscopy (EM) nucleic acid probes (DNA probes) Serologic Assays Indications to perform serologic assays for diagnosis of viral infections are limited. With a few exceptions, paired sera (acute and convalescent) demonstrating seroconversation or four-fold rise are required to establish diagnosis of recent infection. Serologic studies are also usually retrospective because of the need for paired sera. Indications for serology include: diagnosis of infections with nonculturable organisms such as hepatitis viruses determination of immune status to rubella, measles, varicella-zoster, hepatitis B monitoring of immunosuppressed and transplant patients epidemiologic or prevalence of studies 138 3. Virus Isolation In clinical virology, isolation of the virus is the “gold” standard. Traditionally, there are three methods used for isolation of viruses are cell culture, animal inoculation and the use of embryonated eggs. Of these methods, cell culture is most commonly used. 139 BENCH OBJECTIVES FOR DIAGNOSIS OF BACTERIAL SEPSIS REFERENCES: 1. 2. 3. Cumitech #1A Blood Culture II Microbiology and Public Health Laboratory Manual Selected Reference Articles GENERAL OBJECTIVES: The goals of this rotation are to ensure that the trainee is capable of understanding the clinical and technical aspects of laboratory diagnosis of sepsis. The trainee should understand and be able to communicate the following: 1. Pathogenesis of septicemia. 2. Difference between bacteremia and septicemia. 3. Clinical reasons for collection of blood cultures. 4. General principles in determining volume, frequency, and number of blood culture collections in various clinical situations. 5. Role of instrumentation (e.g. central venous or arterial lines) in the development of bacteremia and septicemia. 6. Principles of blood/broth ratios. 7. Procedures for working up of positive blood cultures including notification of preliminary and final results. 8. Role of the medical microbiologist interacting with the attending physician in the care of the patient with positive blood cultures. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Describe the principles, operation, uses, advantages and disadvantages of the following blood culture systems: a. b. c. d. Conventional broth Lysis centrifugation (Isolator) i. Radiometric ii. Bactec - infrared Continuous-monitoring systems i. Bactec - fluorigenic ii. Colorimetric (Bacti/Alert) iii. Other new systems 140 e. f. Pressure (Signal) Bi-phasic media (Roche) 2. Describe the importance of various ingredients of blood culture media including lytic agents, resins, SPS, etc. 3. Describe methods to make early decisions on the presumptive identification and susceptibility of organisms isolated from blood cultures (e.g. rapid methods according to cell morphology, pellets, direct I.D./susceptibility). 4. Understand and recognize difficulties in accurate morphological identification of organisms in Gram stains from blood cultures. 5. Understand when incubation of blood cultures should be extended, when blood cultures should be subcultured, and causes of false positive and false negative blood cultures. 6. Distinguish contaminants from significant pathogens in blood cultures. 141 BENCH OBJECTIVES FOR INFECTIONS ASSOCIATED WITH STERILE BODY FLUIDS REFERENCES: 1. 2. 3. 4. Cumitech #14 Microbiology and Public Health Manual (Koneman) (ASM Manual) GENERAL OBJECTIVES: The goal of this rotation is to ensure that the trainee is capable of understanding the clinical and technical aspects of infections in sterile body sites. The trainee should understand and be able to communicate the following: 1. Outline infections of the central nervous system, and other normally sterile body systems including host-related and virulence-related factors. 2. List common pathogens in (1) 3. Compare and contrast the physical, chemical, and cellular features of bacterial, mycobacterial, fungal, syphilitic, viral and parasitic central nervous system (CNS) infections. 4. As (3) for other sterile body system infections. SPECIFIC OBJECTIVES: At the end of this rotation, the trainee should be able to: 1. Describe the collection, transportation and processing of: CSF synovial fluid pericardial fluid peritoneal fluid vitreous fluid amniotic fluid pleural fluid 2. Describe the characteristics of normal CSF. 3. Correctly interpret direct Gram stains from sterile body fluids, especially CSF. 4. Understand the principles of rapid diagnostic tests, specifically antigen-detection tests. BENCH OBJECTIVES FOR BACTERIAL ANTIGEN DETECTION REFERENCES: 142 1. 2. 3. Cumitech #14 Microbiology and Public Health Laboratory Manual Selected References GENERAL OBJECTIVES: The goals of this rotation are to assure that the trainee understands the principles and application of direct antigen detection for the diagnosis of microbial infections. The trainee should understand and be able to communicate the following: 1. Pathogenic mechanisms that result in the appearance of microbial antigens in body fluids and tissues. 2. Recognize and be able to differentiate clinical situations where antigen detection may be of value for early diagnosis. 3. Timing and collection of appropriate specimens for antigen detection. 4. Interpretation of antigen results for the provision of appropriate treatment of patients. SPECIFIC OBJECTIVES: At the end of the rotation the trainee should be able to: 1. Describe methods for the detection of microbial antigens in body fluids, including; a. b. c. d. Latex agglutination Enzyme immunoassay (EIA) Immunofluorescence PCR 2. Develop criteria to estimate the sensitivity and specificity of each method. 3. Describe the most appropriate methods and specimens for identification of antigens from; a. b. c. d. e. f. Haemophilus influenzae Streptococcus pneumoniae Neisseria meningitidis Escherichia coli Streptococcus agalactiae Cryptococcus neoformans 4. Understand and interpret false positive and negative results. 5. Determine clinical situations and laboratory manipulations which may prevent a positive result. 6. Use other clinical information (e.g. CSF protein, glucose, etc.) as aids to antigen detection for diagnosis of invasive I 143 BENCH OBJECTIVES FOR UPPER AND LOWER RESPIRATORY TRACT INFECTIONS REFERENCES: 1. 2. 3. 4. Cumitech #7A and #10 Microbiology and Public Health Manual (Koneman) (ASM Manual) GENERAL OBJECTIVES: The goals of this rotation are to ensure that the trainee is capable of understanding the clinical and technical aspects of respiratory infections. The trainee should understand and be able to communicate the following: 1. Define the importance of normal flora in the respiratory tract and explain how alterations may lead to infectious diseases. 2. Discuss the basic pathogenic mechanisms of infections diseases of the respiratory tract and associate the virulence factors of the organisms that cause that disease. 3. Given the clinical picture presented and the symptoms, associate the most probable organisms that cause upper and lower respiratory tract infections. 4. Describe the pathogenesis, risk factors and complications associated with these infections as well as the types of specimens collected for diagnosis. 5. Describe the principles and methods of proper specimen collection and transport of respiratory secretions. 6. Determine the acceptability for culture of respiratory specimens. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Discriminate between normal flora and pathogenic microorganisms, based on colonial and Gram-stain morphology. 2. Outline the laboratory diagnosis (to include specimen collection, direct microscopic examination and culture) for specimens from patients with: pharyngitis sinusitis otitis media epiglottitis pertussis bronchitis and bronchiolitis 144 pneumonia empyema 3. Determine the appropriate level of identification and antimicrobial susceptibility testing. 4. Be able to communicate information on clinical utility of antimicrobials used for respiratory infections. 145 BENCH OBJECTIVES FOR GASTROINTESTINAL INFECTIONS CAUSED BY BACTERIA REFERENCES: 1. 2. 3. 4. Cumitech #12 Microbiology and Public Health Manual (Koneman) (ASM Manual) GENERAL OBJECTIVES: The goal of this rotation is to ensure that the trainee is capable of understanding the clinical and technical aspects of infections in gastrointestinal specimens. The trainee should understand and be able to communicate the following: 1. Explain the role of normal microbial flora of the GI tract. 2. Explain the role of pathogenic mechanisms involved in acute bacterial diarrheas. 3. List the infectious bacteria known to cause diarrhea. 4. Give the sources of these infectious agents and describe how they are acquired. 5. Describe the parameters used for presumptive differential diagnosis. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. State the common enteric pathogens. 2. State the differential, selective and enrichment media used in the isolation of enteric pathogens. 3. Identify enteric pathogens from stool cultures recognize possible pathogens on primary culture. perform and correctly interpret screening tests. confirm identity of possible pathogens biochemically and/or serologically. 4. Outline appropriate specimen collection and handling of enteric specimens. 146 BENCH OBJECTIVES FOR URINARY TRACT INFECTIONS REFERENCES: 1. 2. 3. Cumitech #2A. Laboratory Diagnosis of Urinary Tract Infection Microbiology and Public Health Laboratory Manual Selected References (e.g. Stamm) GENERAL OBJECTIVES: The goals of this rotation are to assure that the trainee understands the clinical and technical aspects of laboratory diagnosis of urinary infections. The trainee should understand and be able to communicate the following: 1. Pathogenesis of urinary tract infections. 2. Prevalence of urinary infections in certain age groups and gender populations. 3. Clinical reasons for collection of a urine culture. 4. Methodologies for collection of a urine culture, including timing, volume of culture, etc. 5. Role of instrumentation (e.g. indwelling catheters, in-out catheters, stents, suprapubic catheters, etc.) in the collection, analysis and interpretation of urine cultures. 6. Procedures for working up positive urine cultures including notification of preliminary and final results. 7. Role of the medical microbiologist interacting with the attending physician in the care of the patient with positive urine cultures. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Understand and communicate the limitations of reporting of numerical colony counts. 2. Understand and interpret the quantitation of the urine culture in various patient groups and according to the type of sample collected. 3. Distinguish probable causative agents of infection from contaminants of the urine. 4. Interpret the significance of isolates according to the colony count, type of specimen and relative degree of contamination, if any. 5. Determine the appropriate level of identification and susceptibility testing according to the isolate and colony count. 147 6. Understand the principles, operation, advantages and disadvantages of alternative methods for detection of organisms in urine including: a. b. c. d. Urine Dip Slides Chemiluminescence Leukocyte Esterase and Nitrate Bactometer 148 BENCH OBJECTIVES FOR MISCELLANEOUS INFECTIONS (NON-STERILE BODY SITES) REFERENCES: 1. 2. 3. Manual of Clinical Microbiology Microbiology and Public Health Laboratory Manual Selected References GENERAL OBJECTIVES: The goals of this rotation are to assure that the trainee understands the clinical and technical aspects of cultures from wounds and other non-sterile body sites. The trainee should understand and be able to communicate the following: 1. Mechanisms of infections in non-sterile body sites. 2. Recognize and differentiate pathogens from commensal flora in the culture. 3. Methodologies for collection of specimens from non-sterile body sites, including aspirates, biopsies, etc. 4. Value of anaerobic cultures (see specifics of anaerobic rotation) in the diagnosis of infection from a wound. 5. Procedures for working up cultures from non-sterile body sites, including recognition of circumstances when a preliminary result should be communicated to the attending physician, and when full identification and susceptibility testing should be performed. 6. Role of the medical microbiologist interacting with the attending physician in the care of the patient with a positive wound culture. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Demonstrate skill in interpretation of direct Gram smears. 2. Understand the use of differential and selective media for isolation and identification of pathogens. 3. Understand, select and interpret the results of antibiotic susceptibilities for appropriate agents that may be used to treat pathogens isolated from wounds, etc. 149 4. Identify potential pathogens from these sites, including both usual organisms such as S. aureus, and unusual organisms; i.e., Stenotrophomonas, Acinetobacter, Eikenella, Pasteurella, etc. 5. Identify anaerobes as causative agents in these infections (e.g. Clostridium species, Bacteroides species) - also see Bench Objectives for Anaerobes. 150 BENCH OBJECTIVES FOR BACTERIAL INFECTIONS OF THE GENITAL TRACT REFERENCES: 1. 2. 3. 4. Cumitech #4, #17 Microbiology and Public Health Manual (Koneman) (ASM Manual) GENERAL OBJECTIVES: The goal of this rotation is to ensure that the trainee is capable of understanding the clinical and technical aspects of infections in genital specimens. The trainee should understand and be able to communicate the following: 1. Describe the clinical manifestations produced by the following agents: Neisseria gonorrhea Candida albicans Chlamydia trachomatis Gardnerella vaginitis Treponema pallidum subsp. pallidum Haemophilus ducreyi Trichomonas vaginalis 2. Describe the proper specimen collection and laboratory methods used to diagnose the diseases caused by each of the previously listed organisms. 3. Differentiate between vaginosis and vaginitis. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Define bacterial vaginosis and understand the N-score procedure. 2. List and recognize, by colonial appearance, probable pathogens from commensal flora in the following sites: vagina cervix penis urethra 3. Identify specimens appropriate and inappropriate for N. gonorrhoea culture 4. Identify N. gonorrhoeae: 151 5. conventional methods enzymatic methods coagglutination methods Identify Trichomonas vaginitis: N-score wet preps 6. Outline the method, to include specificity and sensitivity, for direct detection of Chlamydia trachomatis. 152 BENCH OBJECTIVES FOR BACTERIAL INFECTIONS OF THE GENITAL TRACT REFERENCES: 1. 2. 3. 4. Cumitech #4, #17 Microbiology and Public Health Manual (Koneman) (ASM Manual) GENERAL OBJECTIVES: The goal of this rotation is to ensure that the trainee is capable of understanding the clinical and technical aspects of infections in genital specimens. The trainee should understand and be able to communicate the following: 1. Describe the clinical manifestations produced by the following agents: Neisseria gonorrhea Candida albicans Chlamydia trachomatis Gardnerella vaginitis Treponema pallidum subsp. pallidum Haemophilus ducreyi Trichomonas vaginalis 2. Describe the proper specimen collection and laboratory methods used to diagnose the diseases caused by each of the previously listed organisms. 3. Differentiate between vaginosis and vaginitis. SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Define bacterial vaginosis and understand the N-score procedure. 2. List and recognize, by colonial appearance, probable pathogens from commensal flora in the following sites: vagina cervix penis urethra 3. Identify specimens appropriate and inappropriate for N. gonorrhoea culture 4. Identify N. gonorrhoeae: 153 5. conventional methods enzymatic methods coagglutination methods Identify Trichomonas vaginitis: N-score wet preps 6. Outline the method, to include specificity and sensitivity, for direct detection of Chlamydia trachomatis. 154 BENCH OBJECTIVES FOR ANTIMICROBIAL SUSCEPTIBILITY TESTING REFERENCES: 1. 2. 3. 4. Cumitech #25, #6A Microbiology and Public Health Laboratory Manual NCCLS Documents M2 and M7 Lorian: Antibiotics in Laboratory Medicine GENERAL OBJECTIVES: The goals of this rotation are to ensure that the trainee understands the clinical and technical aspects of antimicrobial susceptibility testing. The trainee should understand and be able to communicate the following: 1. Mode of action and primary uses of each class and specific antimicrobial agents. a. b. c. d. e. f. g. h. h. i. Penicillins and their derivatives Cephalosporins - 1st to 4th generation Aminoglycosides Macrolides Tetracyclines Glycopeptides Metabolic inhibitors (e.g. sulphas, trimethoprim) Nucleic acid antagonists (e.g. rifampin, nitrofurantoin, fluoriquinolones) Membrane inhibitors (detergents) Others 2. Mechanisms of microbial resistance to antimicrobial agents. 3. Methodologies used for in vitro and in vivo testing of antimicrobial agents. 4. Correlation between in vitro testing and in vivo response. 5. Role of the medical microbiologist interacting with the attending physician to provide clinically relevant antibiotic susceptibility data. 6. Appropriate issue preliminary antibiotic susceptibility information. 7. Indications for performing antimicrobial susceptibility tests. 8. Develop a program for comparative susceptibility testing of new antimicrobial agents. 9. Describe a rational, cost-effective program for antibiotic susceptibility testing and reporting in a small, medium or large hospital or community setting. SPECIFIC OBJECTIVES: 155 At the end of the rotation, the trainee should be able to: 1. Understand the principles and techniques for antibiotic susceptibility testing, including: a. b. c. d. e. 2. Describe the quality control of antibiotic susceptibility testing, including: a. b. c. d. e. f. g. h. 3. Disk diffusion test (Kirby Bauer) Agar diffusion test (replicator method) Broth dilution (macro and micro) Gradient endpoint (e.g. E-Test) Automated systems (e.g. Vitek, Microscan) Selection of Q.C. strains Maintenance of antibiotic disks, stock, solutions, etc. Preparation of antibiotics for testing Interpretation of NCCLS quality control data Media Inoculum and preparation of inoculum Incubation McFarland standards, etc. Describe the rational development of panels for antibiotic susceptibility testing of the following groups of organisms: a. b. c. d. e. f. Aerobic Gram negative bacilli Enterobacteriaceae Pseudomonads Aerobic Gram-positive cocci (Staphylococci, Streptococci, and Enterococci) Aerobic Gram positive bacilli (Corynebacteria, etc.) Miscellaneous organisms (Stenotrophomonas, Haemophilus, Moraxella, etc.) Anaerobes Yeasts and/or fungi 4. Describe and provide the rationale for cascading of antimicrobial susceptibility results. 5. Describe screening tests for the detection of resistance in various groups of bacteria, including: a. b. c. d. e. f. g. Methods for beta-lactamase testing (e.g. nitrocefin, iodometric, etc.) Oxacillin screening for pneumococci and staphylococci Vancomycin screening for enterococci Screening for extended spectrum beta-lactamase in Enterobacteriaceae Detection of beta-lactamase negative, ampicillin, resistant (BLNAR) trains of Haemophilus Detection of chloramphenicol acetyltransferase (CAT) Other new problems as they become evident 6. Understand the basis for MIC and MBC tests and their interpretation. 7. Describe methodologies for MIC/MBC tests including: 156 a. b. c. d. e. f. 8. Describe the techniques, uses, advantages and disadvantages of methodologies for determination of antibiotic concentrations in body fluids: a. b. c. d. 9. Media Preparation of antibiotic solutions and dilutions Incubation Inoculum preparation, standardization and inoculation McFarland standards Interpretation of data (e.g., skipping, Eagle phenomenon, etc.) Serum bactericidal tests Bioassays Enzyme immunoassays Fluorimetric immunoassays Explain the meaning of susceptible, intermediate and resistant as applied to antimicrobial susceptibility test results. 157 BENCH OBJECTIVES FOR ANAEROBES REFERENCES: 1. 2. 3. Microbiology and Public Health Manual Wadsworth Anaerobic Bacteriology Laboratory Manual ASM Manual. GENERAL OBJECTIVES: 1. Differentiate obligate anaerobes from facultative organisms. 2. Describe how anaerobes, as part of normal flow, initiate and establish infection. anaerobes at specific gastrointestinal tracts) 3. anatomical sites (respiratory tract, skin, genitourinary and Given the clues to an anaerobic infection (signs and manifestations), give the most probable etiologic agent of the following: wound botulism gas gangrene actinomycosis Lung abscess peritonitis 4. Give the bacteriologic indications used to recognize anaerobes as the possible causative agent. 5. Describe the clinical infections associated with the following organisms and how they are acquired and manifested: Clostridium species Anaerobic, non-spore forming, Gram-positive bacilli Actinomyces species Bacteroides species Fusobacterium species Gram-positive cocci Veillonella sp SPECIFIC OBJECTIVES: 1. Describe the laboratory methods of performing and identifying anaerobes: acceptable/unacceptable specimens culture environments isolation media identification systems (to include disc tests) (presumptive and definitive identification). 158 2. Outline the parameters to be used to determine susceptibility testing: when to test antimicrobial agents to be tested quality assurance considerations 3. Describe the acceptable methods for performing anaerobic antimicrobial susceptibility testing. 4. Give the microscopic, colonial morphology and key reactions in tests used to identify the following organisms: C. perfringens C. tetanus C. botulinum Actinomyces israelii Actinomyces odontolyticus Bifidobacterium species Enbacterium species Mobiluncas species Prevotella species Propionibacterium species Bacteroides species Porphyromonas species 159 BENCH OBJECTIVES FOR MYCOLOGY REFERENCES: 1. 2. 3. 4. Kwon-Chung, Bennett: Medical Mycology Rippon: Medical Mycology Microbiology and Public Health Laboratory Manual Koneman, Roberts: Practical Laboratory Mycology GENERAL OBJECTIVES: The goals for this rotation are to assure that the trainee understands the clinical and technical aspects of examination of laboratory specimens for yeasts and filamentous fungi. The trainee should understand and be able to communicate the following: 1. Pathogenesis of different types of fungal infection: a. b. c. d. Superficial Cutaneous or dermatophytic Subcutaneous Invasive 2. Clinical reasons for collection of specimens for fungal examination. 3. Methodologies of collection of specimens including decontamination, appropriate collection vehicles (i.e. scraping, biopsies, aspirates, etc.) 4. Methodologies for transport of fungal specimens. 5. Rapid techniques for primary identification of the presence of fungal elements in clinical specimens. 6. Principles underlying the use of differential incubation temperatures, and length of incubation required to demonstrate different fungal pathogens. 7. Differences between primary and opportunistic infections, and the role of “contaminants” in opportunistic fungal infections. 8. Interaction of the microbiologist with the pathologist in the examination of samples for fungi. 9. Interaction of the medical microbiologist with the attending physician in the care of the patient with a positive fungal specimen. 10. Issues of safety in the mycology laboratory. 160 SPECIFIC OBJECTIVES: At the end of the rotation, the trainee should be able to: 1. Discuss the importance of direct microscopy for examination of fungal elements, including: KOH Calcofluor or Fungifluor Silver stains (e.g. GMS) PAS Giemsa India ink The trainee should be able to discuss the uses and advantages or disadvantages of these direct techniques. 2. Describe the principles, uses, advantages and disadvantages of various primary and selective media for the isolation of fungi. 3. Demonstrate the following methods for the identification of yeasts, including their sensitivity and specificity: a. b. c. d. e. f. g. 4. Demonstrate procedures for the identification of molds including; a. b. c. d. 5. Germ tube Cornmeal with or without Tween Oxgall Assimilation and fermentation tests API 20C Vitek Yeast Card Other methods Scotch tape preparations Tease mounts Slide cultures Differential media Demonstrate and be able to identify where applicable to genus or species level, fungi from the following groups: a. Superficial: Malassezia furfur Hendersonula toruloidea Piedra (Trichosporon beigelii) b. Cutaneous (Dermatophytic): Trichophyton species Microsporum species 161 Epidermophyton species c. Subcutaneous (Demateacious molds) Cladosporium Alternaria Pseudoallescheria boydii Exophiala Fonsecaea d. Dimorphic: Histoplasma capsulatum Blastomyces dermatitidis Coccidioides immitis Sporothrix schenckii Paracoccidioides brasiliensis e. Opportunistic: Aspergillus Fusarium Rhizopus, Mucor, etc. f. Contaminants: Penicillium Scopulariopsis, etc. g. Yeasts: Candida species Cryptococcus 6. Understand and be able to interpret serological tests used for the demonstration of fungal infections including: a. b. c. d. methodologies used - immunodiffusion, EIA, latex agglutination exoantigen tests use of PCR new test modalities 7. Interpret by microscopic and serological results and develop reports that are clinically meaningful to the attending physician. 8. Understand the advantages and disadvantages of anti-fungal susceptibility tests and be able to communicate when such tests may be of value in the management of the patient with a fungal infection. 162 BENCH OBJECTIVES - ENVIRONMENTAL MICROBIOLOGY REFERENCES: 1. 2. Microbiology and Public Health Laboratory Manual Selected articles GENERAL OBJECTIVES: The goals of this rotation are to ensure that the trainee gains an understanding of the role that public health plays in disease prevention, focusing on: 1. The testing and monitoring of drinking, recreational and surface water supplies. 2. The investigation of food- and water-borne disease outbreaks caused by bacterial, viral or parasitic agents. 3. The testing of pharmaceutical agents, blood products and other agents purporting to be sterile. 4. The monitoring of autoclaves to ensure sterility of equipment (i.e. dental instruments, tattoo needles, and syringes). The trainee will also understand the interaction of the Public Health Laboratory with Alberta Health, Alberta Agriculture, Health Protection Branch and Alberta Environmental Protection. The purpose of epidemiological investigations and the importance of the interaction of public health personnel, the Medical Officer of Health and the private physicians will be reviewed. SPECIFIC OBJECTIVES: 1. Understand the significance of the presence or recovery of “indicator” organisms in water samples. 2. Understand the importance of submitting clinical specimens (feces, vomitus, serum), as well as a complete history, when investigating disease outbreaks. 3. Understand the procedure for toxin detection in cases of infant or food-borne botulism. 4. Understand that the recovery of organisms from food and water sources may involve the concentration of large volumes of sample, and that special pre-enrichment and selective enrichment methods are ofter required. 5. Have an awareness of the increasing numbers of pathogens such as Salmonella from sources including snakes, iguanas and hedgehogs. 6. Understand the “EPI” protocol for infectious disease outbreaks. 163 ROTATION SPECIFIC OBJECTIVES - VIROLOGY GENERAL OUTLINE: 1. Total duration of this rotation will vary according to the season. 2. The rotation objectives would include hands-on supervised experience through processing one or more known positive sample(s), - previously tested by the technologists and reported to the user (discard specimens) in both serology and isolation areas. 3. A set of 2-3 “unknowns” - positive specimens (viral suspension or clinical specimens) will be provided to the rotating trainee to be worked on individually. The trainee will keep a log of all procedures performed and record all results for each step as they arise in addition to final result. A flow chart-style task/project design should be a starting point of this assignment. The results and workbook will then be presented to the preceptor (or designate) for review and discussion. 4. A fixed time will be set aside for laboratory based and clinical consultation in the field of virology, done periodically throughout the rotation period with the trainee and the preceptor (or designate). This period is of great importance, since it will be a basis for development of graded responsibility, serve as a tool for evaluation of cognitive skills as well as attitudes, and monitor trainee’s progress. Proposed format - informal laboratory/clinical rounds with virologist or designate, 3-4 times a week. 5. Material to be covered in the rotation is contained in rotation-specific objectives; however, any additional clinical/laboratory experience beyond the objectives is an asset and should be added on as needed and deemed appropriate by the preceptor and/or trainee. 6. An evaluation sheet will be filled out by the preceptor and the resident half-way (mid-rotation) and at the end of rotation period. 164 2A. SPECIMEN SELECTION FOR MAJOR CLINICAL SYNDROMES Summary of appropriate specimen collection sites and timing. The information below should be used as a general guideline. Agent-specific information is presented in the virus isolation chapters. Agent Specimen of Choice Time of Collection Adenovirus Throat swab/wash, rectal swab/stool, urine urine During symptomatic disease Chlamydia Cervical/urethral swab During symptomatic disease C. difficile toxin Stool During symptomatic disease Cytomegalovirus Urine, throat swab/wash, buffy coat During symptomatic disease Enterovirus Throat swab, CSF, stool/rectal swab First week of symptoms Herpes simplex Vesicle fluid/swab, throat/mouth swab, vaginal swab First 3 days of lesion Hepatitis A Serum, stool, liver, kidney First 8 days of symptoms Influenza Throat/NP wash or swab, BAL First 3 days of symptoms Measles Throat swab, urine, blood First 2 days of symptoms Mumps Throat swab, urine, blood First 7 days of symptoms Parainfluenza Throat/NP wash or swab First 3 days of symptoms RSV NP wash/aspirate/swab, throat swab First 3 days of symptoms Rhinovirus NP wash/swab First 2 days of symptoms Rotavirus Stool First 4 days of symptoms Rubella Throat swab, stool, urine First 4 days of symptoms Varicella-Zoster Vesicle fluid/swab, lesion swab First 2 days of symptoms Summary of appropriate specimen collection sites associated with various clinical presentations. This information is provided as a general collection guide. Agent-specific information is presented in the virus isolation chapters. Clinical Presentation Specimen of Choice Other Specimens Bronchitis/Bronchiolitis Nasopharyngeal swabs, washes, and aspirates Broncheoalveolar lavage Colds, Upper Respiratory Tract Infections Nasopharyngeal swabs, washes, and aspirates Throat swabs Croup Nasopharyngeal swabs, washes or aspirates Throat swabs Exanthems Vesicle swab and/or fluid Gastroenteritis Stool Rectal swab Influenza syndrome Nasopharyngeal swabs, washes, and aspirates, sputum Throat swabs or throat washes Meningitis Cerebrospinal fluid (CSF) Throat swab, stool, or rectal swab Pharyngitis Nasopharyngeal swab, wash, or aspirates, throat wash or swab Pneumonia or Lower Respiratory Tract Infections Nasopharyngeal or tracheal aspirates or washes, broncheoalveolar lavages 165 Nasopharyngeal or throat swabs 2B. SPECIMEN SELECTION FOR MAJOR CLINICAL SYNDROMES Specimens for virus isolation and direct detection Source or clinical symptoms and common etiologic agenta Upper respiratory Rhinovirus Influenza virus Parainfluenza virus Adenovirus Enterovirus Cytomegalovirus Epstein-Barr virusb Reovirus Lower respiratory Specimen source for virus isolation and direct detection Clinical Biopsy tissue Naospharyngeal swab, nasal wash (throat Tonsil, lymph node swab) Endotracheal aspirate, bronchial wash, bronchoalveolar lavage (sputum) Lung, bronchus, trachea Lesion swab, lesion fluid Multiple organs Influenza virus Respiratory syncytial virus Parainfluenza virus Cytomegalovirus Chlamydia pneumoniae Mycoplasma pneumoniae Vesicular lesions Herpes simplex virus Varicella-zoster virus Enterovirus Exanthemas Herpesvirus 6 Parvovirus (B19)b Enterovirus Rubeola (measles) virus Rubella virus Rickettsia spp.b Central nervous system Enterovirus Herpesvirus family Arbovirus Posttransplantation syndromes Herpes simplex virus Cytomegalovirus Epstein-Barr virus Herpesvirus 6 Congenital anomalies Cytomegalovirus Herpes simplex virus Rubella virus Varicella-zoster virus Enteritis and diarrhea Rotavirus Enteric adenovirus Astrovirus Calicivirus (Norwalk agents) Nasopharyngeal swab (throat swab, stool) CSF, nasopharyngeal swab Brain Throat swab (urine) Transplanted organ Nasopharyngeal swab, urine Affected organ(s) Stool Colon aSpecimens b in parentheses are secondary choices. Diagnosis by serology, rarely by isolation or direct detection. 166 3. CELL CULTURE SYSTEMS - AN OVERVIEW Common cell lines: species origin, tissue origin, cell morphology Primary MK, RK (HEK, GPE, CE and other) Passaged cell strain or line MRC 5, Hep-2, HeLa, Vero, KB, Hel (BSC-1, RK-13, BHK-21, A549, HDF and other) Cell culture preparation and maintenance: Media/solutions: MEM, Hanks BSS, etc. Incubator, roller drum apparatus Contaminants: bacterial/viral/mycoplasma Selection of appropriate/relevant cell lines Cell lines indicated for processing of routine* specimens Source Respiratory Expected Virus Cell Line RSV Influenza A/B AGMK RHMK Adenovirus HEP2 Enterovirus VZV, HSV Skin / mucous membranes VZV, HSV AGMK Coxsackie A Eye CSF HSV AGMK Adenovirus HEP2 Enterovirus AGMK HSV RHMK Mumps HEP2 AGMK Stool Adenovirus RHMK Enterovirus HEP2 Suspected CMV MRC-5 shellvials HEL * For details, see virology manual 167 4. INOCULATION OF CELL LINES Inoculation techniques Enhancement of viral infectivity Postinoculation centrifugation Biochemical enhancement (theory only) 5. EXAMINATION OF CELL CULTURES FOR VIRAL ACTIVITY/VIRAL ISOLATION AND IDENTIFICATION (METHODS) CPE in susceptible culture (HSV, VZV) Hemadsorbtion/hemagglutination (Influenza, parainfluenza, measles) Embryonated egg inoculation (VZV, influenza) Spin-amplification shell vial assay (CMV) Virus inclusions (SSPA) (demo only - slides/kodachromes) Confirmation of viral identity: Antibody-based techniques (Fluorescent Ab) Electron microscopy DNA hybridization (Refer to Molecular Diagnostics for theory, demonstration and practical experience) PCR (Refer to Molecular Diagnostics for theory, demonstration and practical experience) Neutralization tests (theory only) 6. DIRECT VIRAL ANTIGEN DETECTION: COLLECTION AND PROCESSING Principle of immunofluorescent/immunoenzyme staining - EM, FA, EIA, other: PCR, DNA probes (theory; see appendices for diagrams) 168 Specimen handling, preparation and processing: Vesicular lesion smears nasopharyngeal smears Cell sediments smears (buffy coats, tissues, etc.) Electron microscopy Sensitivity Specimen selection: diagnostic utility (diseases/organisms) Laboratory procedures: Thin section (theory) Negative staining technique(s) Agar diffusion Immune EM Review of photomicrographs of major viruses 6A. PRACTICAL EXAMPLES EM for: Rotavirus, Norwalk FA testing for: HSV, VZV, RSV, Adenovirus, influenza, parainfluenza PCR for: HSV, Enteroviruses, VZV (to be done in Molecular Diagnostics) 7. DIAGNOSTIC TESTS FOR IDENTIFICATION OF SPECIFIC VIRAL INFECTIONS Herpes VZV RSV Parainfluenza Mumps Rhinovirus Hepatitis A, B, C, D, E Enteroviruses HIV/HTLV Other (Arbovirus, Hantavirus) CMV EBV Influenza Measles Rubella Adenovirus Rotavirus Rabies Pox 169 8. VIRAL SEROLOGY Specimen collection, transport and storage Basic concepts review: screening vs. confirmatory testing, sensitivity/specificity PPV, NPV - low/high prevalence population Method overview Discussion and demonstration: Neutralization EIA IFA Western blot (Refer to Molecular Diagnostics for theory, demonstration and practical experience) PCR (Refer to Molecular Diagnostics for theory, demonstration and practical experience) Discussion only: CFT FIAX Hemagglutination assays Hemolysis inhibition 8A. AVAILABLE TESTS - OBSERVE/PERFORM IgG/IgM antibody detection for: CMV, EBV, VZV, HSV HIV, HTLV Hepatitis A, B, C Rubella, measles, mumps Parvovirus 8B. SERODIAGNOSIS OF MAJOR VIRAL INFECTIONS Discussion of: methods, advantages/disadvantages, clinical utility/interpretation, limitations of the procedures 170 HIV Hepatitis Rubella Other (EBV, HSV, measles, influenza/parainfluenza) 9. TEST OPTIMIZATION IN VIROLOGY e.g.: 10. RSV for DFA - when to test (seasonality) HSV in patients with lesions: culture vs. serology INTERPRETATION OF VIROLOGY RESULTS: Laboratory problem solving/troubleshooting Blood sample crushed in a centrifuge Mycoplasma contamination of tissue cultures Positive control reading as (-) in an automated EIA test Inadequate specimen collected and/or wrong timing Cell culture ? contaminated with an animal virus Clinical consultations: PBL - style illustrative mini case discussions: Pregnant contact of varicella Newborn with multiple congenital abnormalities IV drug abuser donating blood Needlestick injury in a lab worker MVA victim as a potential organ donor Returning traveller with fever and jaundice Toddler with fever and maculopapular rash Elderly bronchitic in the settling of a large influenza epidemic Infant in severe respiratory distress 171 Outbreak of diarrhea in a daycare centre/nursing home Otherwise healthy young male with encephalitis Student with sore throat and lymphadenopathy Teenager bitten by a stray dog Returning missionary (Africa) with fever, DIC and multiorgan failure Painful corneal ulcer in a cold sore sufferer Advice to pathologist working in autopsy suite 11. UNKNOWN SPECIMENS The trainee will keep a log of all procedures performed and record all results for each step as they arise in addition to final result. A flow chart-style task/project design should be a starting point of this assignment. Viral Isolation Identify virus in suspension or patient’s sample (source provided) Serology Identify a positive serum (a short clinical history provided) 172 MODIFIED OBJECTIVES - DERMATOLOGY (Short Rotation) 1. LABORATORY ORIENTATION AND SPECIMEN RECEIVING A. Introduction to diagnostic laboratory and staff B. Basic principles of laboratory safety: Universal precautions Basic understanding of WHMIS General safety and protective measures Use of biosafety cabinets Use of disinfectants C. Overview of specimen collection, transport and storage Types of specimens Sites of collection Quantity and timing Collection devices D. Initial sorting and handling of clinical specimens: Swabs Tissues/sterile fluids Aspirates (pus, wound drainages, etc.) Specimens for anaerobe investigation Bloods Urines Sputums Stools Other (if not listed above) E. Laboratory techniques: Proper handling of biological specimens and culture media Preparation of smears for direct microscopic examination Sterile inoculation techniques Incubation of cultures (atmosphere/temperature) F. Specimen rejection criteria and policies 173 G. Initial processing of selected specimens / choice of culture media: Wound swabs Purulent exudates (including anaerobic cultures) Tissues Skin scrapings for Mycology (may be covered in Mycology rotation) Burns Intravenous catheters (IV tips) H. Direct staining of clinical specimens: Gram smear Ziehl-Neelsen/Auramine-rhodamine FA staining (any example - may be covered in virology rotation) Calcofluor white (may be covered in mycology or parasitology rotation) 2. PARASITOLOGY A. Brief overview of methods: Stool preservatives Concentration techniques Routine (stool) stains, tissue stains and microscopy: Wet mount Trichrome or Iron Hematoxylin, Kinyoun Giemsa Calcofluor white, GMS B. Demonstration of parasites of skin and subcutaneous tissues: Arthropods (Lice, mites/scabies, fleas, ticks, myiasis) Amoebae (free living and E. histolytica) Leishmania sp Strongyloides stercoralis and hookworm larvae Filariae (discuss skin snip procedure) C. Self study: parasitic diseases involving skin and subcutaneous tissue Dermatitis Leishmania sp Schistosoma spp Strongyloides stercoralis Cutaneous larva migrans Ankylostoma brasiliense, canis other Filaria spp Loa loa 174 Onchocerca volvulus Gasterophilus sp Sarcoptes scabiei, mites, lice Rash/Hives Ascaris lumbricoides Schistosoma spp Trichinella spiralis Toxoplasma gondii Trypanosoma gambiense/rhodesiense Abscess Filaria spp E. histolytica Nodule/Swelling Calabar swelling (L. loa) Chagoma (T. cruzi) Taenia solium Onchocercoma (O. volvulus) Coenurosis (Multiceps multiceps) Echinococcus granulosus Sparganum sp Hypoderma sp Dermatobia sp Winterbottom’s sign (Trypanosoma gambiense/rhodesiense) Trophic Skin Changes, Various L. donovani S. haematobium W. bancrofti Brugia malayi O. volvulus Prediculus humanus Cutaneous Ulcer L. tropica complex L. brasiliensis T. gambiense/rhodesiense E. histolytica Dracunculus medinensis 3. MYCOLOGY (Superficial mycosis) 175 A. Specimen selection B. Brief overview of methods: Direct microscopy: KOH/wet mount, PAS, calcofluor white Overview of selective media and growth characteristics Germ tube test for yeast identification Brief overview of (relevant) commercial kits C. Demonstration of direct microscopical appearance and colonial morphology Candida spp (including, but not limited to C. albicans) Geotrichium Rhodotorula Sporothrix schenkii Dermatophytes (including a selection of unknowns to be identified from microscope or Kodachrome slides) Aspergillus sp Penicilium spp Other as deemed necessary/interesting D. Self study: Saprophytic vs. opportunistic fungi Diagnosis of superficial mycoses 4. GENERAL BACTERIOLOGY A. Function and primary purpose of media Blood agar (BAP) Chocolate MacConkey Mannitol salt Brain heart infusion (BHI) Supplemented peptone broth (SPB) PEA B. Primary purpose of biochemicals/tests Catalase Coagulase (slide and tube) DNA se Bacitracin Optochin Bile solubility Strep grouping Bile esculin PYR 176 Oxidase Satellitism ALA Indole Urea C. Brief overview of Vitek automated identification system (demo only) D. Common microbiology tests/procedures: lab workup and interpretation Demonstration of plates with mixed flora and how pure cultures are obtained. Demonstration of common potential pathogens and contaminants/normal flora of the wound, abscesses, skin/subcutaneous tissue specimens, central and peripheral line tips, burns, etc. (swabs, aspirates, tissues, other). Interpretation and assessment and significant quantitation/colony count of common pathogens on the primary plates. Correlation of direct smear results with colony types and numbers of primary culture plates. Demonstration of correct recognition and assessment of amounts of common aerobic and anaerobic organisms. E. Review of isolation and identification procedures for selected (special emphasis) organisms: Staphylococci Streptococci Hemophilus influenzae Corynebacteria Pseudomonas Enterobacteriaceae 177 F. Self study: Surface skin flora and its relative pathogenicity in skin and soft tissue infections. Agents of wound, superficial skin infections, cellulitis and fasciitis/myositis /myonecrosis (aerobic and anaerobic). Special protocols: quantitative burn biopsies, semiquantitative line tip cultures. 5. ANAEROBIC BACTERIOLOGY A. Review of anaerobic specimen collection and transport systems: diagnostic value of different specimen types (e.g.: swabs vs. aspirates or tissue biopsies, specimens unsuitable for anaerobic cultures). Syringe/needle tube/vial swab/plastic jacket system bio-bag/plastic pouch B. Anaerobic cultivation systems - demonstration: Anaerobic jar/holding jar Anaerobic glove box C. Function and primary purpose of media: Anaerobic BAP PEA Anaerobic BHI BBE Other D. Identification of major anaerobic pathogens: Clostridium sp (Nagler reaction) Bacteroides sp Actinomyces sp Anaerobic cocci E. Gas liquid chromatography for bacterial identification (demonstration) F. Self Study: Relationship of bacteria to oxygen: oxygen tolerance/redox potential, anaerobe-aerobe synergism. Normal human anaerobic flora mouth/oral cavity 178 upper respiratory tract skin/mucous membranes other sites: gastrointestinal tract, genital tract, urethra, etc. Indications for cultures: problems, pitfalls and limitations of anaerobic bacteriology. Human anaerobic infections of exogenous and endogenous origin, with particular emphasis on: superficial skin/wound infections cellulitis myonecrosis actinomycosis animal/human bites pseudomembraneous colitis antibiotic-associated diarrhea 179 Medical Microbiology Rotation Schedule This is the general guide for Medical Microbiology Rotations for General Pathology Residents. Topics and timing may be altered as required to fit both resident and laboratory schedules. Week General Area/Topic Location Number 1 General Orientation – Maria Ackney UAH 2. Specimen Receiving UAH 3 Quality Control /Culture Media UAH 4 General Bacteriology Pods UAH 5 General Bacteriology Pods UAH 6 General Bacteriology Pods UAH 7 Blood cultures UAH 8 Enterics UAH/PL 9 Antimicrobial susceptibility Testing I UAH 10 Anaerobes UAH 11 Genitals UAH 12 Infectious Diseases Pod UAH 13 Review and examination UAH End of First Rotation 14 Mycobacteriology PL 15 Mycology UAH/National Centre 16 Reference Microbiology UAH/PL 17 Viral Culture/Direct Detection UAH/PL 18 Viral serology UAH/PL 19 Viral serology UAH/PL 20 Chlamydia/Mycoplasma UAH/PL 21 Molecular Diagnostics UAH/PL 22 Parasitology UAH 23 Infection Prevention and Control UAH 24* Community Microbiology DynaLIFE 25* Community Microbiology DynaLIFE 26 Review and Examination UAH End of Second Rotation * Residents usually spend the last 6 months of their program at DynaLIFE and are again exposed to Microbiology during that time. They also attend regular microbiology sessions given by RP Rennie as Royal College Examination preparation In addition, the residents are expected to attend the following seminars, rounds, etc. Daily: Infectious diseases plate rounds. Monday AM (optional): Infectious Diseases Journal Club Tuesdays: Academic half day – microbiology seminars and central didactic teaching series. Thursdays: Infectious Diseases Rounds Fridays (usu.) Topic sessions with RP Rennie Graded Responsibility for service on-call is established after the resident has been in the laboratory for 4 – 5 weeks. A senior microbiologist is always available for back-up. 180 ROTATION SPECIFIC OBJECTIVES IN NEUROPATHOLOGY General Pathology Residents Selection: Mandatory Site: University of Alberta Hospital Preceptors: Dr. E.S. Johnson and Dr. L. Resch Length of rotation: Four weeks Prerequisites: PGY 2 Specific Objectives: MEDICAL EXPERT To have a general knowledge of the macroscopic neuroanatomy of the brain and spinal cord, including blood supply. To be familiar with the microscopic anatomy of major neuroanatomic structures of the brain. To acquire in depth knowledge of the major classes of tumors of the nervous system. To be acquainted with the macroscopic and microscopic pathologic features of cerebral infarcts and ischemia, cerebral hemorrhages of different causes, infections of the nervous system, craniocerebral trauma, and common neurodegenerative diseases. To demonstrate an understanding of the appropriate use of basic histologic techniques, including immunohistochemistry and electron microscopy, in the examination of the nervous system. To understand the applicability of enzyme histochemistry in the examination of muscle biopsies, and to recognize the microscopic features of denervation, muscular dystrophy, myopathy and inflammatory myopathies and vasculopathies. To perform basic dissections in the removal of the brain and spinal cord, and to prepare these tissues for subsequent study. To be familiar with standard safety precautions in the removal of the brain in situations of infectivity (i.e., HIV infection, prion disease). To be able to dissect the brain and spinal cord in accordance with standard neuropathologic techniques, and select appropriate tissues for microscopic examination. To be able to handle neurosurgical specimens for frozen section, preparation of smears, and interpretation of these techniques to render a verbal diagnosis. To be able to examine macroscopically neurosurgical specimens and to appropriately sample these specimens for microscopy. To gain confidence and develop an interest in examining tissue from the nervous system. To understand the methodological differences required in examining neuropathological specimens compared to general surgical pathology. function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care establish and maintain clinical knowledge, skills and attitudes appropriate to their practice perform a complete and appropriate assessment of a patient use preventative and therapeutic interventions effectively demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic seek appropriate consultation from other health professionals, recognizing the limits of their expertise COMMUNICATOR To be able to formulate reports based upon macroscopic and microscopic observations for autopsy and neurosurgical specimens, with suitable clinicopathologic correlations. 181 Assist in the continuing education of physicians and other members of the hospital staff by participating in conferences and case presentations To act as consultants to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient. develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals accurately convey relevant information and explanations to colleagues and other professionals develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families convey effective oral and written information about a medical encounter COLLABORATOR To demonstrate the ability to advise on the appropriateness of obtaining histological, cytological and autopsy specimens and to advise on further appropriate investigations including infection control and safety. participate effectively and appropriately in an interprofessional healthcare team effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and lifelong learning MANAGER participate in activities that contribute to the effectiveness of their healthcare organizations and systems manage their practice and career effectively allocate finite healthcare resources appropriately serve in administration and leadership roles, as appropriate HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to neuropathological processes As a member of the interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health respond to individual patient health needs and issues as part of patient care respond to the health needs of the communities that they serve identify the determinants of health of the populations that they serve promote the health of individual patients, communities and populations acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports Develop and implement a personal continuing educational strategy Apply the principles of critical appraisal to sources of medical information Contribute to the development of new knowledge through research Participate in rounds, conferences and teaching sessions SCHOLAR 182 maintain and enhance professional activities through ongoing learning critically evaluate information and its sources, and apply this appropriately to practice decisions facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate contribute to the creation, dissemination, application, and translation of new medical knowledge and practices PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion Practice medicine in an ethnical manner and with a sensitivity to diverse patient and co-worker populations Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner Demonstrate commitment to excellence and ongoing professional development demonstrate commitment to excellence and ongoing professional development demonstrate a commitment to their patients, profession, and society through ethical practice demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation demonstrate a commitment to physician health and sustainable practice Outline of Rotation: 1. 2. 3 4. 5. 6. 7. 8. 9. Residents are expected to undertake the reading assignments as listed in “Educational Materials”. Residents are to attend and participate as necessary in Neuropathology-Braincutting Conferences, Muscle Biopsy Conference, and Neuroscience Rounds. Residents are expected to be present at all frozen sections and to gross neurosurgical specimens under supervision of attending neuropathologist. Residents will attend sign-out of neurosurgical specimens, and prepare reports on assigned cases. Residents will review muscle biopsy specimens with neuropathologist. Residents will participate in dissection and blocking of autopsy brains, and review microscopy and dictate reports on assigned cases. Residents will undertake with attending neuropathologist autopsies on neuropathology cases and complete these cases. Residents are expected to learn techniques of removal of the brain on all cases coming to autopsy. Residents will review teaching cases in slide boxes with neuropathologists. Educational Materials: Suggested reading 1. Burger, P.C., Scheithauer, B.W. Tumors of the Central Nervous System, Atlas of Tumor Pathology, Third Series, Fascicle 10, AFIP, 1994. 2. Kleihues, P., Cavenee, W.K. Pathology and Genetics of Tumors of the Nervous System, International Agency for Research for Cancer, Lyon, 2000. 3. Gray, F., DeGirolami, U., Poirier, J., Escourolle and Poirier’s Manual of Basic Neuropathology, 4 th edition, Butterworth Heinemann, 2004. 4. Parent, A. Carpenter’s Human Neuroanatomy, 9th edition, Williams and Wilkins, Baltimore, 1996 (Chapters 1,2, and 4) 5. Ellison, D., Love, S., et al. Neuropathology, 2nd edition, Mosby Ltd., 2004. 6. Graham, D.I., Lantos, P.L. Greenfield’s Neuropathology, 6th edition, Arnold, London, 1997. 7. Bigner, D.D., McLendon, K.E., Bruner, J.M. Russell and Rubinstein’s Pathology of Tumors of the Nervous System, 6th edition, Arnold, London, 1998. 183 Residents are expected to read references 1 or 2, assigned chapters in references 3 and 4, and be able to use as resources for further depth of reading references 5, 6, and 7. Conferences 1. Neuropathology Conference, weekly (Friday, 10:00 – 11:00 a.m.) 2. Neuroscience Clinical Conference, weekly (Friday, 9:00 – 10:00 a.m.) Evaluation: 1. 2. On-going daily oral examination of the resident. Assessment of ability to make correct microscopic diagnosis, and quality of completed reports. 3. Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 184 ROTATION SPECIFIC OBJECTIVES PEDIATRIC AND PERINATAL PATHOLOGY CYTOGENETICS AND MOLECULAR CYTOGENETICS Selection: Sites: Preceptors: Teachers: Length of Rotation: Prerequisites: Mandatory. University of Alberta/Stollery Children’s Hospital; Royal Alexandra Hospital. Dr. Atilano Lacson/Dr. Consolato Sergi Dr. Atilano Lacson Dr. Consolato Sergi Dr. Nenad Lilic Dr. Suzanne Chan Five weeks. PGY 3 OR higher. OBJECTIVES: MEDICAL EXPERT Function effectively as pathology consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered diagnostic care. Establish and maintain clinical knowledge, skills and attitudes appropriate to Pediatric and Perinatal Pathology, Cytogenetics and Molecular Cytogenetics. Perform a complete and appropriate assessment of gross and histologic aspects of pediatric and perinatal patient specimens. Demonstrate proficient and appropriate use of procedural diagnostic skills, including triage of tissues for specialized studies, including Cytogenetics and Molecular Cytogenetics. Seek appropriate consultation from other pathologists, recognizing the limits of their expertise. Utilize all knowledge databases to bear to help with the diagnostic efforts and dilemmas that he/she encounters. Demonstrate sufficient scientific curiosity to convey new information to colleagues, superiors and the medical community locally and internationally, when possible. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied laboratory workers and, where appropriate, with patients and families. Accurately elicit and synthesize relevant information and perspectives of clinical colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on diagnostic issues and problems; plans and discusses solutions accordingly with colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in interprofessional diagnostic and clinical healthcare teams 185 Effectively work with other colleagues and superiors within the Department, other health professionals and human resource experts to prevent, negotiate, and resolve interprofessional conflict. Participate in clinical-pathologic discussions that contribute to the effectiveness of the healthcare organizations and systems. Manage their practices and careers in a goal-directed fashion to recognize and/or create opportunities for advancement in a progressive manner. Use diagnostic resources effectively and allocate finite healthcare resources appropriately by streamlining diagnostic algorithms and balancing this with opportunities for learning. Serve in administrative and leadership roles, when called upon to do so, or during unforeseen events, as appropriate. MANAGER HEALTH ADVOCATE Respond to individual patient diagnostic needs and issues as part of patient care promptly, appropriately, and respectfully. Respond to the larger pathology diagnostic needs of the pediatric communities that they serve. Identify the determinants of health of the pediatric populations that they serve. Promote the health of individual children, pediatric communities and pediatric populations and their caregivers. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of pathology diagnostic reports. SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. 186 Outline of Rotation: Rotation Duration Site Pediatric and Perinatal Surgical and Autopsy Pathology 4 weeks University of Alberta / Stollery Children’s Hospital Royal Alexandra Hospital At the end of this rotation, the learner will be able to: 1. Discuss the gross and microscopic pathological characteristics of commonly encountered benign and malignant neoplasms in children of various age groups. 2. Describe the features of various congenital syndromes leading to malformations based on pathogenetic mechanisms where possible. 3. Illustrate the pathophysiology of commonly encountered conditions in the fetal and neonatal period, and their clinical effects. Cytogenetics and Molecular Cytogenetics 1 week University of Alberta Cytogenetics Laboratory At the end of this rotation, the learner will be able to: 1. Integrate a sound understanding of the principles behind cytogenetic and molecular cytogenetic testing and interpretation and how these principles are utilized to guide genetic counseling. 2. Generate a morphogenetic differential diagnosis of cases based on major features. 3. Describe possible molecular pathogenetic mechanisms of commonly encountered inherited or sporadic diseases. 187 Educational Materials: 1. Pediatric Pathology Textbooks housed in pathologists’ offices. 2. Resident Library 3. University of Alberta Library Services 4. Online journals and websites for additional learning materials. 5. Proceedings of recent national and international conferences. 6. Glass and photograph slide collections of teaching cases with individual pathologists. Evaluation: Overall assessment is based on a standard resident evaluation form distributed through Webeval. To be approved by RPC 188 ROTATION SPECIFIC OBJECTIVES SURGICAL PATHOLOGY Selection: Site: Co-preceptors: Length of Rotation: Prerequisites: Mandatory DynaLIFE DX Diagnostic Laboratory Services Core Lab Dr. Carolyn O’Hara Minimum of 4 weeks Minimum experience of a PGY3 resident. Only one resident in AP and one in Cytology (ie two individuals) will be accommodated at DynaLIFE at any one time. Specific Objectives: MEDICAL EXPERT Develop expertise in the examination, description, accessioning and record-keeping, dissection and sampling of gross surgical pathology specimens for pathologic examination Develop expertise in the pathologic examination of surgical specimens, including the use of special stains and immunoperoxidase phenotyping techniques Develop expertise in the accurate and timely compilation of surgical pathology reports to communicate pathological findings and make relevant clinical assessment and appropriate recommendations with regard to the subsequent management of patients Develop the ability to consult with both clinical and pathology colleagues to effectively assist in the management of patients Develop critical reading skills to evaluate and incorporate new information as published in the scientific pathologic literature, and to share with and teach such information to colleagues and peers and develop and adopt appropriate continuing educational habits Develop leadership, managerial and communication skills related to the effective and efficient running of a pathology laboratory Develop an understanding of evidence-based medicine, and good laboratory practice Become familiar with laboratory accreditation procedures and appropriate quality assurance and quality control mechanisms, including acceptance of and participation in peer review Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice Perform a complete and appropriate assessment of a patient Use preventative and therapeutic interventions effectively Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic Seek appropriate consultation from other health professionals, recognizing the limits of their expertise COMMUNICATOR Assist in the continuing education of physicians and other members of the staff by participating in conferences and case presentations Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed) 189 Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals Accurately convey relevant information and explanations to colleagues and other professionals Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families Convey effective oral and written information about a medical encounter COLLABORATOR Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens and to advise on further appropriate investigations Collaborate with colleagues at all levels in the pathology laboratory, particularly in respect of consultation, peer review and continuing professional development Participate effectively and appropriately in an interprofessional healthcare team Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life Allocate finite health care and health education resources effectively to optimize patient care and life-long learning Develop skills in the management of technical and support staff in the laboratory Participate in activities that contribute to the effectiveness of their healthcare organizations and systems Manage their practice and career effectively Allocate finite healthcare resources appropriately Serve in administration and leadership roles, as appropriate HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to pathological processes As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health Respond to individual patient health needs and issues as part of patient care Respond to the health needs of the communities that they serve Identify the determinants of health of the populations that they serve Promote the health of individual patients, communities and populations Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports SCHOLAR Develop and implement a personal continuing educational strategy Apply the principles of critical appraisal to sources of medical information Contribute to the development of new knowledge through research Participate in rounds, conferences and teaching sessions Develop learning techniques and skills to present information in written and oral examinations Maintain and enhance professional activities through ongoing learning 190 Critically evaluate information and its sources, and apply this appropriately to practice decisions Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion Practice medicine in an ethnical manner and with a sensitivity to diverse patient and co-worker populations Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner Demonstrate commitment to evidence-based medicine, excellence and ongoing professional development Demonstrate commitment to excellence and ongoing professional development Demonstrate a commitment to their patients, profession, and society through ethical practice Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation Demonstrate a commitment to physician health and sustainable practice Outline of Rotation: Rotations will commence with orientation and familiarization of the resident with the laboratory layout, and introduction of the resident to the pathologists and staff in the DynaLIFE laboratory The resident will be familiarized with work routines in the DynaLIFE laboratory The resident will spend time in the histology processing lab to enable familiarization with procedures in the histology laboratory regarding the reception, accession, gross examination, dissection, processing, cutting and staining of sections The resident will be integrated into the routine work schedules and will be allocated grossing and reporting duties, and will be encouraged to rotate for periods of tutoring with each of the DynaLIFE pathologists An emphasis will be placed on the resident developing appropriate communication and professional collaborative skills with all support staff and pathologists at DynaLIFE Residents will be encouraged to develop self sufficiency and an ability to formulate a considered pathological opinion and to present that opinion for evaluation to the supervising pathologist Emphasis will be placed on timely accurate reporting with an effort to maintain an average turnaround time of 24 hours per specimen Residents are expected to make an forty minute educational presentation of a topic of current interest during the rotation at DynaLIFE Educational Materials: The DynaLIFE library and pathologists are well equipped with current textbooks and many journal which are available for use by residents 191 Each DynaLIFE pathologist has a multihead teaching microscope enabling simultaneous examination of slides by consulting pathologist and resident An office dedicated for the use of residents is equipped with a computer workstation and high speed internet connection, a good quality binocular microscope, a dictaphone and telephone A multihead microscope with video monitors is available for peer review meetings held every two weeks A learning centre with computer projection facilities is available for resident and staff presentations and scientific meetings DynaLIFE staff includes pathologists with a wide range of pathology expertise and a willingness to teach Evaluation: Overall assessment is based on the Uof A pathology resident evaluation form The final evaluation will as a rule be discussed with the resident who will be encouraged to respond to and discuss any adverse comment Informal, oral weekly evaluation will provide regular feedback on the appropriateness of the resident’s activities An open and honest atmosphere of critical appraisal will prevail. Residents will have the opportunity to share problems and areas of difficulty with either or both of the preceptors if necessary or desired Overall assessment is based on a pathology resident evaluation form distributed through Webeval. July 2007 192 ROTATION SPECIFIC OBJECTIVES SURGICAL PATHOLOGY Selection: Mandatory Site: Misericordia Community Hospital Preceptor: Dr. John Danyluk Length of Rotation: Three to four weeks minimum Prerequisites: Previous rotation in Breast and Urologic Pathology at the Misericordia Hospital. Specific Objectives: MEDICAL EXPERT Gross and report a variety of surgical pathology specimens appropriate to level of training. Participate in frozen section coverage. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient’s medical history relevant to diagnostic surgical pathology. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Assist in the continuing education of physicians and other members of the staff by participating in conferences and case presentations. Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient. Accurately convey relevant information and explanations to colleagues and other professionals. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals verbally and in the form of surgical pathology reports. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. COLLABORATOR Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens and to advise on further appropriate investigations. Collaborate with colleagues at all levels in the pathology laboratory, particularly with respect to consultation, peer review and continuing professional development. Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. 193 MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life. Allocate finite health care and health education resources effectively to optimize patient care and life-long learning. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to pathological processes. As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs. Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. Apply the principles of critical appraisal to sources of medical information. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethical manner and with a sensitivity to diverse patient and co-worker populations. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Ddemonstrate a commitment to physician health and sustainable practice. Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner. Demonstrate commitment to excellence and ongoing professional development. 194 Outline of Rotation: The resident is assigned cases by the preceptor and other pathologists on a weekly rotation. These weekly rotations include gross dissection, microscopic evaluation and dictation of reports, and sign-out with the assigned staff pathologist. Residents will also participate in frozen sections during the weekly rotation. Educational Materials: General and specialty text books. Collection of teaching material for review, glass slides, etc. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 195 ROTATION SPECIFIC OBJECTIVES SURGICAL PATHOLOGY Selection: Mandatory Site: Royal Alexandra Hospital Preceptor: Dr. Robert West Length of Rotation: 6 weeks Prerequisites: PGY2 Specific Objectives: MEDICAL EXPERT Demonstrate the ability to handle complex surgical specimens including complex genitourinary, gastrointestinal, gynecological and thoracic cases. Residents should be able to appropriately gross these cases and independently prepare and dictate completed reports prior to reviewing with a supervising pathologist. The resident should demonstrate appropriate knowledge of appropriate ancillary investigations (i.e., histochemical, immunohistochemical stains etc.) Demonstrate the ability to perform a complete post mortem examination, with appropriate full description and diagnosis at gross and microscopic levels. Resident must be able to interpret their findings in the light of the clinical history and communicate these in an effective written and oral fashion. They must be completely familiar with the rules governing consent for post mortem examination and the type of case that should be reported to the coroner or the medical examiner’s office. Demonstrate the ability to perform frozen sections. The resident should develop the ability to appropriately interact with physicians requesting frozen sections to determine the appropriateness of the request as well as to determine pertinent clinical information. The resident should develop the skills to appropriately sample specimens. The resident will be expected to develop the skills to allow for technical processing of the specimens (cutting sections and performing stains) without technical support. Finally the resident will develop a level of competency to properly interpret the frozen sections. Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient’s medical history relevant to diagnostic surgical pathology. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Assist in the continuing education of physicians and other members of the staff by participating in conferences and case presentations. Act as consultant to clinical colleagues on the interpretation and relevance of pathological findings, with particular regard to their significance in the management of the patient. Convey effective oral and written information about a medical encounter. Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals verbally and in the form of surgical pathology and autopsy reports. 196 Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. Demonstrate the ability to advise on the appropriateness of obtaining histological and cytological specimens and to advise on further appropriate investigations. Collaborate with colleagues at all levels in the pathology laboratory, particularly with respect to consultation, peer review, and continuing professional development. MANAGER Utilize time and resources effectively to balance patient care, budget restrictions, professional expectations and personal life. Allocate finite health care and health education resources effectively to optimize patient care and life-long learning. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Serve in administration and leadership roles, as appropriate. HEALTH ADVOCATE Identify the important determinants of health affecting patients pertaining to pathological processes. As a member of an interdisciplinary team of professionals responsible for patient health, the resident will assist in regularly evaluating laboratory practices and test selections to determine that they meet community needs. Recognize and reinforce to the public and to the medical profession the essential contribution of laboratory medicine to health. Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Develop and implement a personal continuing educational strategy. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices by participating in research, rounds, conferences and teaching sessions.. Apply the principles of critical appraisal to sources of medical information. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. 197 Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. PROFESSIONAL Deliver the highest quality of care with integrity, honesty and compassion. Practice medicine in an ethical manner and with a sensitivity to diverse patient and co-worker populations. Exhibit appropriate professional behavior and perform duties in a dependable and responsible manner. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Demonstrate commitment to excellence and ongoing professional development. Outline of Rotation: Senior AP (R4 and R5) and GP residents (R4 and R5) will rotate through the RAH on a 6 week basis. During each week of rotation, the resident will be expected to gross surgical specimens for two half days, attend and perform frozen sections with a staff pathologist for one half day, be responsible to cover the autopsy service for a day and under the supervision of the attending pathologist prepare reports on the majority of cases which they have been exposed to. Depending on the abilities and interest of the residents, they will also be able to access smaller biopsy material for review with the attending pathologists. The residents will be expected to be available from 8:00 am until 5:00pm daily unless it is their academic half day, they are off work sick or they have made special arrangements with the supervising pathologist. When scheduled to assist the attending pathologist with frozen sections, the resident will have to ensure that they are available in the hospital and available by pager to attend the frozen sections. This will require the residents to be on site at 8:00 am when they are scheduled for morning frozens. Residents will be expected to learn how to properly sample material in order to chose frozen section blocks. They will also be expected to learn how to perform the technical skills for handling such specimens (i.e. cutting and staining of slides) and learn how to appropriately interpret frozen sections. Finally they will be expected to interact with surgeons and gynecologists requesting the frozen sections to determine appropriate clinical information and to determine the appropriateness of performing the frozen section. When scheduled on the autopsy service they will be expected to perform no more then one autopsy in a given day. The resident will be expected to review the chart for appropriate clinical information and ensure proper consent. With the assistance of a pathology assistant they will perform an external examination, internal examination dissection and assessment of the organs prior to reviewing the organs and other pertinent findings with the supervising pathologist. They will be expected to prepare a provisional diagnostic report and to dictate the clinical history, external and internal examinations within 24 hours. They will be expected to review the microscopic slides with the supervising pathologist and complete the report prior to the end of their rotation. The resident will be expected to cut specimens for two half days and to cut any frozen section cases in which they participated. The supervising pathologist and the resident will then decide an appropriate number of cases which the resident will be required to examine microscopically. The workload will therefore vary depending on the capability of the resident. Residents will be given the opportunity to examine smaller biopsy specimens known as pool cases which they were not involved with grossing. The residents will be expected to dictate completed reports on the more straight 198 forward cases prior to review by the supervising pathologist. For more complicated cases, the resident will discuss the cases with the supervising pathologist and appropriate additional studies will be decided upon prior to dictating any report. It is the expectation that the resident attend all academic half days during their rotation unless prevented from doing so because of health reasons or vacation. Finally it is the expectation that each resident perform a half an hour formal presentation to all of the attending staff pathologists once during the rotation. The presentation should be centered around an interesting case which they encountered during their rotation. The resident will also be expected to present at the monthly journal clubs. Educational Materials: The CH is presently replacing the pathologists’ microscopes with new microscopes, all of which possess sidearms. The CH has also promised to buy a new multiheaded microscope for the resident’s room. The residency teaching program has made a commitment to provide two microscopes for use by residents on the site. The residency program has also made a commitment to provide a computer and a C-phone for use by the residents. The department subscribes to the College of American Pathologists PIP in Surgical Pathology. A small library is available within the resident’s room. Residents are also able to borrow pathologists’ textbooks. There is a digital camera present within the department but no other audiovisual equipment or computers are available for preparing presentations for rounds. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. February 2007 199 ELECTIVE ROTATIONS 200 ROTATION SPECIFIC OBJECTIVES IN DERMATOPATHOLOGY Selection: Elective. Site: DynaLIFE core laboratory. Dr. Schloss’s office, College Plaza. Preceptor: Dr. E Schloss. Prerequisites: PGY3/4/5. Length of rotation: Four weeks. (Minimum two weeks). Educational Objectives: MEDICAL EXPERT Develop and improve diagnostic skills for the recognition of common skin conditions both inflammatory and neoplastic. Understand the principles and applications of special diagnostic testing to dermatopathology.Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. MANAGER Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. 201 HEALTH ADVOCATE Respond to individual patient health needs and issues as part of patient care. Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. SCHOLAR PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: Review current cases with preceptor at DKML and his office. If time permits, review archival material. Attend Dermatology Rounds weekly. Educational Materials: Suggested reading – Histopathology of the Skin. Lever, Walter F. Conferences – Dermatology Rounds. Clinical Material – Current and archival cases. 202 Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 203 ROTATION SPECIFIC OBJECTIVES FOR PULMONARY PATHOLOGY Selection: Site: Elective Grey Nuns Hospital Royal Alexandra Hospital Preceptor: Dr. I. Sin Prerequisites: PGY 3/4 Length of rotation: Minimum two weeks. Specific Objectives: MEDICAL EXPERT Function effectively as consultants, integrating all of the CanMEDS Roles to provide optimal, ethical and patient-centered medical care. Establish and maintain clinical knowledge, skills and attitudes appropriate to their practice. Perform a complete and appropriate assessment of a patient. Use preventative and therapeutic interventions effectively. Demonstrate proficient and appropriate use of procedural skills, both diagnostic and therapeutic. Seek appropriate consultation from other health professionals, recognizing the limits of their expertise. COMMUNICATOR Develop rapport, trust and professional relationships with other physicians and allied health care workers and patients and families (as needed). Accurately elicit and synthesize relevant information and perspectives of patients and families, colleagues and other professionals. Accurately convey relevant information and explanations to colleagues and other professionals. Develop a common understanding on issues, problems and plans colleagues and other professionals to develop a shared plan of care in the best interests of patients and families. Convey effective oral and written information about a medical encounter. COLLABORATOR Participate effectively and appropriately in an interprofessional healthcare team. Effectively work with other health professionals to prevent, negotiate, and resolve interprofessional conflict. Participate in activities that contribute to the effectiveness of their healthcare organizations and systems. Manage their practice and career effectively. Allocate finite healthcare resources appropriately. Serve in administration and leadership roles, as appropriate. Respond to individual patient health needs and issues as part of patient care. MANAGER HEALTH ADVOCATE 204 Respond to the health needs of the communities that they serve. Identify the determinants of health of the populations that they serve. Promote the health of individual patients, communities and populations. Acquire appropriate QA/QC knowledge to ensure patient safety and accuracy of medical reports. SCHOLAR Maintain and enhance professional activities through ongoing learning. Critically evaluate information and its sources, and apply this appropriately to practice decisions. Facilitate the learning of patients, families, students, residents, other health professionals, the public, and others, as appropriate. Contribute to the creation, dissemination, application, and translation of new medical knowledge and practices. PROFESSIONAL Demonstrate commitment to excellence and ongoing professional development. Demonstrate a commitment to their patients, profession, and society through ethical practice. Demonstrate a commitment to their patients, profession, and society through participation in profession-led regulation. Demonstrate a commitment to physician health and sustainable practice. Outline of Rotation: The resident shall be expected to: Perform gross examination and dissection of lung specimens and demonstrate ability to handle fresh lung specimens appropriately. Perform microscopic examination and report the lung cases. Perform intra-operative consultation and frozen sections. Review cases in the lung teaching files. Demonstrate the ability to correlate pathologic findings with clinical and radiologic findings. Demonstrate the ability to use special stains and immunohistochemical studies appropriately. Demonstrate the ability to communicate effectively with the clinicians and radiologists. Attend and participate, if required, in clinical rounds. Understand the importance of a multidisciplinary approach. Demonstrate the ability to appraise relevant literature and application. Understand the bioethical and medicolegal issues. Understand the importance of quality assurance and risk management. Function as an effective team member with the hospital and laboratory staff. Demonstrate professional maturity. Demonstrate ability to perform self-assessment. Demonstrate self-motivation and ability to do self-directed learning. Evaluation: Overall assessment is based on a pathology resident evaluation form distributed through Webeval. April 2006 205 ROTATION SPECIFIC OBJECTIVES LABORATORY MANAGEMENT TRAINING T The general pathology resident should develop a basic knowledge of the following, particularly as they apply to larger hospitals: A. The administrative structure and relationships of a hospital and laboratory, including: (i) Hospital organizational structure (ii) Medical staff organizational structure (iii) Laboratory organizational structure (iv) Hospital administrator's role (v) Laboratory director's role (vi) Technical director's (laboratory manager, chief technologist) role (vii) Medical staff and hospital committees (viii) Hospital and laboratory accreditation. B. The administrative structure and relationships with the Laboratory, including: (i) Laboratory organizational structure (ii) Laboratory director's role (iii) Technical director's (laboratory manager, chief technologist) role (iv) Staff pathologist's role (v) Ph.D. clinical chemist's, clinical microbiologist's role (vi) Pathologist-technologist relationships. C. Laboratory management decisions, including: (i) Personnel selection and evaluation - hiring and firing (ii) Job descriptions and their preparation (iii) Personnel problems (iv) Union agreements and legal considerations (v) Budget preparation and control (vi) Canadian workload units (vii) Staffing requirements (viii) Workflow patterns with and between laboratory departments (ix) Program and facility planning (x) Equipment selection and evaluation (xi) Quality control procedures (xii) Data processing - including effective methods for filing, retrieval and distribution of specimens and results. D. Managerial Skills: Other areas in which specific managerial skills are required of an general pathologist in the direction of a laboratory include: ability to determine those procedures required on-site vs. those that can be referred in the light of medical needs and human and financial resources available understanding of technical staff qualifications related to level of training ability to select and develop new technical methods preparation and maintenance of procedure manuals 206 - - preparation and maintenance of user manuals for physicians and nursing staff ability to conduct an effective quality control program provision of an efficient external referral mechanism for tests not performed in the hospital laboratory maintenance of an appropriate safety program which will ensure the protection of personnel, patients and the laboratory environment from fire, toxic or explosive chemicals and gases, infectious agents and radiation hazards an understanding of the federal government safety standards for the use of low-level radioisotopes in in-vitro test procedures adoption of an efficient method for reporting of results and for their indexing which will allow for rapid reporting and data retrieval the ability to advise on budget preparation with an understanding of the costs involved in laboratory operation, i.e. personnel, supplies, and capital equipment. 207 PROGRAM EVALUATION OF RESIDENTS Throughout the program, each resident will be evaluated regularly by means of on-going evaluation of overall performance (completed through an on-line evaluation system) and by formal examinations. The evaluations will be discussed with the residents and their results will be retained on file. Evaluations in the final year will form the basis of the Final In-training Evaluation Report to the Royal College. A resident may appeal any evaluation report considered unfair. The resident has the right to appeal to: (a) The Program Director, Departmental Chair and Associate Dean for Postgraduate Education. (b) The appeals process defined by the University of Alberta Residency Training Programs. The first year of the residency program is probationary and will be subject to a more vigorous evaluation procedure. Evaluations will be done at the end of specific rotations and at least every three months. Formal examinations will be held throughout the year. Oral, written and practical examinations are conducted twice a year. Oral examinations are structured similar to those of the RCPSC. The content of the oral examination will be determined by the level of training of the resident. The written examination in the Fall will also be mirrored on the RCPSC Fellowship Examination and is designed primarily to test senior residents preparing for the forthcoming Fellowship Examination. It is expected that more junior residents will not do as well as their senior colleagues but there is an expectation on the part of the program that performances will improve in subsequent years. The written examination in the Spring will be the Resident In-Service Examination of the American Society of Clinical Pathologists (ASCP). Despite the emphasis on evaluation, we are confident that you will enjoy your training experience, and hope that you will accept the periodic evaluation in the constructive spirit in which it is intended. 208 RESIDENT EVALUATION OF THE PROGRAM AND FACULTY Resident feedback is important in providing information about the effectiveness of our training programs and will be used to improve the program where necessary. Each resident completes an evaluation of their rotation immediately upon completion of it (through an on-line evaluation system). When the system flags a low performance for a resident, this is brought immediately to the attention of the Program Director. The residents are also encouraged to bring any issues or concerns directly to the Program Director, if they prefer. In addition to evaluating rotations, residents are asked to evaluate the teaching faculty on an annual basis (again, through an anonymous on-line evaluation system). These evaluations are similar to those used in the undergraduate program. The numerical scores for each teacher are forwarded to the Academic Chair and the Program Director for their review. Both the numerical score and the narrative comments are distributed to the individual teacher. It is expected that resident evaluation of the program will be conducted in a collegial and constructive fashion. Each year the residents recognize a member of the faculty who has excelled in teaching, through the Teacher of the Year Award. Award recipients are recognized at the department annual Resident and Graduate Student Research Day. 209 POLICY OF THE GENERAL PATHOLOGY RESIDENCY TRAINING PROGRAM REGARDING RESIDENTS WORKING AS PHYSICIAN EXTENDERS 1. Eligibility The Resident must be in his 2nd year of training or later and meet the requirements laid out by the Royal College of Physicians and Surgeons of Alberta The Resident’s application for licensure must be reviewed and approved by the Residency Program Director The resident must have CMPA coverage as specified by the Royal College of Physicians and Surgeons of Alberta guidelines 2. Time Commitment To be negotiated between the Resident, Residency Program Director, and Residency Program Committee The Resident must maintain a satisfactory level of academic standing and performance within the program At no time shall physician extender work interfere with residency training commitments At no time shall physician extender work impose extra duties or time constraints onto fellow residents The time committed to physician extender work must not interfere with normal service and educational responsibilities of the Resident the following day. The after hours coverage as part of the educational experience, taken together with shifts as a physician extender, must not breach the PARA/CAHCA agreement. 3. Any violation of the above may result in suspension of licensure Type of Training Wherever possible, an attempt should be made to engage in physician extender work that will provide as much relevance as possible to the discipline within which the Resident is engaged in training at that time 4. Monitoring There will a review of physician extender activities semi-annually by the employer and the Residency Program Director 210 There will be a review of the Resident’s academic standing, performance, and physician extender licensure semi-annually by the Residency Program Director November 2004 211 VACATION Residents are entitled to twenty days vacation annually. Vacation time must be approved by the Program Administrator who is to be notified of the leave six months in advance. A resident may take all of his/her vacation entitlements at one time or divided into two blocks of 1 & 3 weeks or 2 & 2 weeks. Vacation time for the academic year must be taken within that year. This is in accordance with the current PARA agreement. 212 FACULTY CODE OF CONDUCT The Code of Conduct document of the Department of Laboratory Medicine and Pathology, Faculty of Medicine and Dentistry can be accessed at: http://www.med.ualberta.ca/deansoffice/code_of_conduct_2.pdf 213