POLAR, NONPOLAR AND SOLUBILITY
advertisement

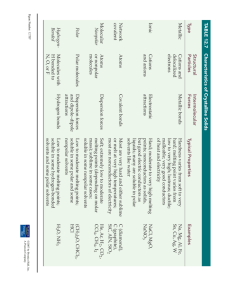
POLAR, NONPOLAR AND SOLUBILITY Introduction: All compounds can be classified as ionic, polar or nonpolar. In this experiment you will attempt to determine a “rule” that can be used to determine what type of substances (solutes) will dissolve in other substances (solvents). Specifically, you will add six solutes to two different solvents and determine if they dissolve. The solutes you will use are the following: Urea** (polar); Iodine** (nonpolar); Ammonium chloride** (ionic); Lighter fluid** (nonpolar); Copper (II) sulfate** (ionic); and Sodium chloride (ionic). The two solvents you will try to dissolve them in are water (polar) and hexane** (nonpolar). Purpose: In this experiment, I will attempt to determine a “general rule” that describes the solubility of solutes in polar and nonpolar solvents. Materials: 6 Test tubes, distilled water, hexane** and various solutes. Acetone** will be used to rinse your glassware in the final stages of cleaning up. (** means that these substances have hazards with skin, eyes or respiratory system, so please use care when using them) Terms: Soluble – the ability of something to dissolve in something else. Procedure: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. You must prepare your own data table to record your results. Have the solutes listed on the left side and the solvents listed on the top. In the data table, be sure to indicate if the solute or solvent is polar, nonpolar or ionic. Using a 10-mL graduated cylinder, measure 5 mL of distilled water and add it to a medium-sized test tube. Do this to 5 more test tubes. In each test tube add one of the six solutes. If a solute is a liquid, add 10-20 drops. If it is a solid, add a match head-sized sample using a spatula (this is a VERY small amount!!!). After adding the solute, mix the contents of each test tube by tapping the bottom of the tube while firmly holding the top. Your teacher will demonstrate this for you. Determine how well each solute dissolves in water. Use this key to record your observations: S – soluble; SS – slightly soluble; NS – not soluble. If you need help, be sure to ask! Discard the contents of each test tube in the “water waste beaker” in the fume hood. Clean out the test tubes using water and a brush – then rinse each with ACETONE to dry them. Repeat steps 1-5 using hexane as the solvent in place of the water. Make sure all of the water is out of your test tubes before you use the hexane + solids. Discard the contents in the “hexane waste beaker” in the fume hood. Wash glassware with a brush and water. Then do a final rinse with acetone. Leave your test tubes upside down to dry. Questions: 1. What types of compounds were more soluble in water than hexane? 2. What types of compounds were more soluble in hexane than water? 3. What characteristic do the solutes that are soluble in water share in common? 4. Determine the general rule of solubility of solvents. 5. What type of compound (polar, nonpolar or ionic) do you think oil is? Why? 6. Did any solutes produce unexpected results? Describe those results.