Procedure - UNM Health Sciences Center
advertisement

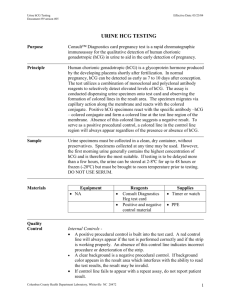
Applies To: All HSC Hospitals, CRTC, or UNMH Component(s): Responsible Department: Rapid Response Laboratory Title: Point of Care Testing: Urine Pregnancy Test Patient Age Group: ( ) N/A ( ) All Ages ( ) Newborns Procedure ( ) Pediatric (X ) Adult DESCRIPTION/OVERVIEW 1. PURPOSE 1.1. The QuickVue One-Step hCG Urine test is a sensitive immunoassay for the qualitative detection of human chorionic gonatotropin (hCG) in urine for the early detection of pregnancy. 2. PRINCIPLE 2.1. Human Chorionic Gonadotropin is a hormone produced by the placenta shortly after implantation. Since hCG is present in the urine of pregnant women, it is an excellent marker for confirming pregnancy. 2.2. The QuickVue test uses a monoclonal antibody specific to the beta subunit of hCG in a single-step technology to accurately detect hCG. REFERENCES QuickVue hCG package insert, 05/2005 AREAS OF REPONSIBILITY 1. POCT QuickVue hCG testing can be done on any inpatient or outpatient at University Hospital on receipt of an order from a physician or nursing staff designate. 2. Staff who have satisfied initial and annual competency requirements may perform the testing. PROCEDURE 1. SUPPLIES 1.1. Quidel QuickVue Urine hCG test cassettes 1.2. Quidel Disposable Pipette 1.3. Commercially available liquid Positive and Negative urine pregnancy quality controls 2. ORDERING INFORMATION 2.1. Test cassettes are ordered through purchasing. 2.2. Quality control solutions are ordered through purchasing. 3. STORAGE 3.1. Store test kits at room temperature, out of direct sunlight. 3.2. Kit contents are stable until the expiration date printed on the outer box carton. 3.3. Store QC solution per manufacturer’s guidelines. 3.4. QC material will expire per manufacturer’s guidelines. 4. QUALITY CONTROL 4.1. Internal Quality Control Features Title: Point of Care Testing: Urine Pregnancy Test Owner: Point of Care Testing Coordinator Effective Date: Page 1 of 5 4.1.1. The internal quality control must be documented for every patient or external quality control sample tested. 4.1.1.1. Acceptable methods for documentation of internal QC include using a Point of Care department provided log sheet or by utilizing Ad Hoc charting in Cerner Millennium when performing patient testing. 4.1.2 The appearance of a blue procedural Control Line is an internal positive control. This indicates that sufficient sample fluid was added for capillary flow to occur and the correct procedural technique was used. If this line does not develop, the test result is invalid. 4.1.3. A clear background in the test result window is an internal negative control. If the test has been performed correctly, the background should be white to light pink within 3 minutes and not interfere with the reading of the test result. 4.2. External Quality Control Testing 4.2.1. When the testing department opens a new box of cassettes it will be QC’d with a positive and negative external quality control. 4.2.1.1. Notify the Point of Care Department if the external quality control fails. 4.2.2. Obtain two cassettes from the box. 4.2.3. Apply three drops of positive external quality control to the sample window. 4.2.4. Read the results at three minutes. The test MUST appear positive. 4.2.5. Repeat with the negative control. The test MUST appear negative. 4.2.6. Document results on the appropriate quality control log including any repeats or corrective actions taken. 4.2.7. Document on the box of cassettes that QC was performed and the date. 4.2.8. The cassettes may be used for patient testing as long as the external and internal quality controls performed properly. 4.2.9. If the external or internal quality controls do not perform properly, repeat once. If the quality control still does not perform properly contact the Point of Care Testing Office at 2-0980 do not perform patient testing. 5. PATIENT MANAGEMENT 5.1. Patient Identification 5.1.1. Patient identification may be the patient’s name, date of birth, medical record number, financial number or assigned trauma alert name. 5.1.2. The University of New Mexico Health Sciences Center (UNMHSC) does not recognize patients’ social security numbers as a patient identifier. 5.2. Patient Preparation 5.2.1. The operator must describe to the patient the purpose and steps of the procedure before testing can begin. 6. SPECIMEN COLLECTION AND HANDLING 6.1. Standard precautions apply to all point of care tests. 6.2. Testing personnel should handle all patient samples as per the Bloodborne Pathogen Exposure Plan. 6.3. Collect freshly voided urine in a clean, dry container. 6.4. First morning specimens generally contain the highest concentrations of hCG and are recommended for early detection of pregnancy. However, any urine specimen is suitable for testing. Title: Point of Care Testing: Urine Pregnancy Test Owner: Point of Care Testing Coordinator Effective Date: Page 2 of 5 7. SPECIMEN LABELING 7.1. Specimens should be tested immediately at the patient’s bedside and discarded. 7.2. If immediate, bedside testing is not possible the sample should be labeled with two unique identifiers. 7.3. Patient identification may be the patient’s name, date of birth, medical record number or assigned trauma alert name. 7.4. UNMHSC does not recognize patients’ social security numbers as a patient identifier. 8. PATIENT TESTING 8.1. Remove the QuickVue test cassette from the foil pouch just before use and place it on a clean, dry, level surface. 8.2. Using one of the disposable pipettes supplied, collect sample and add 3 drops (125 µL) of urine to the round sample well on the test cassette. The test cassette should not be handled or moved until the test is complete and ready for reading. 8.3. Wait three minutes and read. Some positive results may be seen sooner. 9. INTERPRETATION OF RESULTS 9.1. Positive: Any pink-to-red test line (T) along with a blue control line (C) is a positive result for the detection of hCG. Specimens containing as low as 25mIU/mL hCG will yield positive results. 9.2. Negative: A blue Control Line (C) and no pink test line (T) is a negative result. 9.3. Invalid: The test result is invalid if a blue control line (C) is not visible at 3 minutes. If this happens, retest using a new sample and a new test cassette. Contact the point of care testing office at 2-0980. NORMAL RANGE: hCG is not normally detected in the urine of healthy men and healthy non-pregnant women. 10. RESULT REPORTING 10.1. Patient results should be immediately recorded in the permanent medical record. 10.2. Results that appear to be inconsistent with patient therapy or condition should be viewed as questionable and the test should be repeated by the laboratory reference method. 11. INTERFERING SUBSTANCES AND LIMITATIONS 11.1. The Quidel QuickVue urine hCG is for use in the qualitative detection of hCG in urine. 11.2. Test results must always be evaluated with other data available to the physician. 11.3. While pregnancy is the most likely reason for the presence of hCG in urine, elevated hCG concentrations unrelated to pregnancy have been reported in some patients. Conditions other than normal pregnancy may be associated with detectable hCG, including, for example ectopic pregnancy or molar pregnancy. Patients with trophoblastic and non-trophoblastic disease may have elevated hCG levels; therefore, the possibility of hCG secreting neoplasms should be eliminated prior to the diagnosis of pregnancy. 11.4. hCG may remain detectable for a few days to several weeks after delivery, abortion, natural termination or hCG injections. Title: Point of Care Testing: Urine Pregnancy Test Owner: Point of Care Testing Coordinator Effective Date: Page 3 of 5 11.5. Abnormal pregnancies cannot be diagnosed by qualitative hCG results. The above conditions should be ruled out when diagnosing pregnancy. 11.6. Early pregnancy associated with a low level of hCG may show color development after the 3-minute procedure time. If a negative result is obtained but pregnancy is suspected, hCG levels may be too low or urine may be too dilute for detection. Another specimen should be collected after 48 – 72 hours and tested. If waiting 48 hours is not medically advisable the test result should be confirmed with a quantitative hCG test. CLIA Classification Use If definitive, how will results be used? Waived Definitive Results will determine confirmation of pregnancy. DEFINITIONS None SUMMARY OF CHANGES Replaces POCT Urine Pregnancy Testing, Date in use 3/13/06 KEY WORDS Pregnancy test, urine pregnancy test, urine pregnancy Title: Point of Care Testing: Urine Pregnancy Test Owner: Point of Care Testing Coordinator Effective Date: Page 4 of 5 RESOURCES/TRAINING Point of Care Testing, 2-0980 Resource/Dept Internet/Link DOCUMENT APPROVAL & TRACKING Owner Item Contact Point of Care Testing Coordinator Date Consultant(s) Tricore Reference Laboratories Committee(s) Point of Care Testing Advisory Committee [Y or N/A] Nursing Director Medical Director Human Resources Finance Legal Official Approver [Name], Chief Nursing Officer Glynnis Ingall, MD, PhD; Rapid Response Laboratory [Name], HR Administrator, [UNMH or UNM] [Name, Title], [UNMH or HSC] [Name, Title], [UNMH or HSC] [Name, Title, Area] [Y or N/A] [Y or N/A] [Y or N/A] [Y or N/A] [Y or N/A] Y [Day/Mo/Year] Official Signature Approver (Optional) Signature [Day/Mo/Year] Effective Date Origination Date Issue Date [Day/Mo/Year] [Month/Year] [Day/Mo/Year] Clinical Operations P&P Coordinator ATTACHMENTS None Title: Point of Care Testing: Urine Pregnancy Test Owner: Point of Care Testing Coordinator Effective Date: Page 5 of 5 Approval