POCT Pregnancy test - BioSign Instructions for use
advertisement

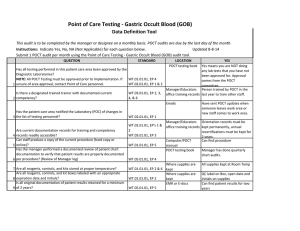
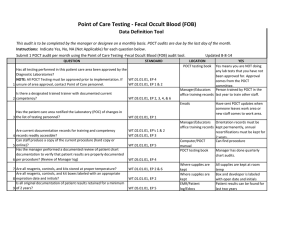
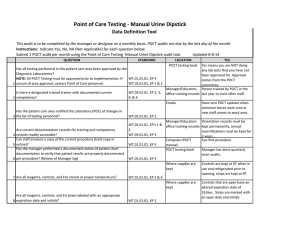
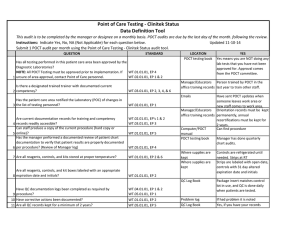
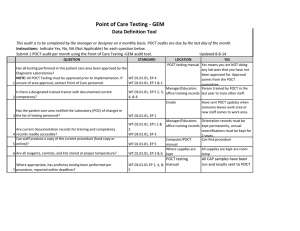
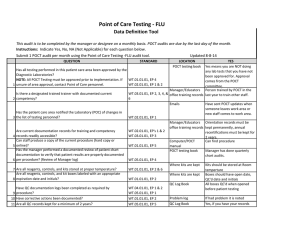
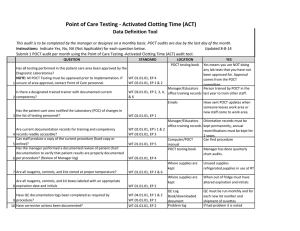
BLOOD SCIENCES DEPARTMENT OF CLINICAL BIOCHEMISTRY Title of Document: BioSign® Instructions for Use Q Pulse Reference No: BS/CB/POCT/SOPS/19 Authoriser: Francesca Mills Version NO: 2.0 BioSign® hCG - New One Step Pregnancy Test Kit Instructions for Use Test Procedure 1. Label the device with the patient name. 2. Fill the dropper with the sample (without air bubbles). 3. Holding the dropper in a vertical position, add 3 drops of sample into the sample well. 4. Read the result at 3-5 minutes. Do not interpret the result after 5 minutes. Interpretation of results POSITIVE = TWO pinkish-purple lines, one each at the Test (T) and Control (C) position. NEGATIVE = ONE pinkish-purple line at the Control (C) position. INVALID = No lines visible or a test line only. Repeat with a new cassette. Any urine sample is appropriate but the first morning urine specimen is optimal for the detection of early pregnancy. The urine should not be too dilute. BioSign® hCG - New One Step Pregnancy Tests have a sensitivity of 25 mIU/ml enabling detection of hCG concentration before the day of expected menstruation. For product queries and to order further kits POCT Team Ext: 38380 or email POCT@nbt.nhs.uk For more detailed instructions please refer to: POCT BioSign® New One Step Pregnancy Test SOP (Q Pulse Reference No: BS/CB/POCT/SOPS/21) Pack insert included in every box of BioSign® hCG – One Step Pregnancy Test kit. Active date: 06/08/2014 Page 1 of 1