Unit 6 – Synthesising Organic Compounds
advertisement

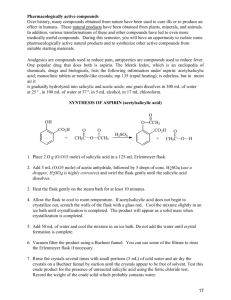
Unit 6 – Synthesising Organic Compounds Practical 1 Synthesis of Aspirin Aim: You will prepare aspirin using salicylic acid (alcohol) and acetic anhydride (acid) using a phosphoric acid catalyst. You will then establish the melting point of the aspirin and purity of the final product if required. Finally the percentage yield of the product will be established. H3PO4 The following reaction is classified as an Esterification reaction. Esterification Reaction The name given to a reaction when two chemical substances (typically an acid and an alcohol) react to form an ester. During this reaction, the alcohol group (-OH group in Salicylic acid) is substituted with the acid (anhydride group) to form an ester (Acetyl salicylic acid). Health and Safety Make sure that your lab coat is fastened at all times. Make sure that you wear gloves and safety goggles at all times. Part 1 of this experiment must be completed within a fumehood. Part 1- Synthesis of Aspirin (To be carried out in school) 1. Weigh out 3g of Salicylic acid and place in a 250ml conical flask. 2. Add 6.0ml of acetic anhydride to the flask. 3. Carefully add 5-10 drops of 85% phosphoric acid to the flask and swirl gently to mix. Phosphoric acid acts as a catalyst. 4. Heat the mixture to approximately 75°C using a heating mantle or Bunsen burner for 10mins. Do not heat the mixture above 75 °C. 5. Cautiously add 20 drops of distilled water. The hot liquid reacts violently with the cold water. 6. Add 20ml of distilled water and cool in an ice-bath. At this point crystals should appear. 7. If this does not happen, scratch the side of the flask with a glass rod or diamond pen to induce crystallisation. 8. Weigh a piece of filter paper and record your results. 9. Set up a Buchner Funnel and insert filter paper. Filter paper Air out 10. Wet the filter paper with distilled water. 11. Filter the solid aspirin using the Buchner Funnel. 12. Wash the crystals using 3ml of chilled water. 13. Dry the crystals and filter paper using an oven overnight at 37°C. 14. Weigh the solid and filter paper and record your results. 15. The solid should be transferred to a universal container and taken to the University on 4th March to establish its purity. Part 2- Establishing purity of product (To be completed in the University) 1. This section will be carried out on your samples of aspirin made in the classroom. Be careful not to spill your sample! 2. Measure the melting point of your aspirin. (The technique below will be demonstrated to you). 3. Place a small amount of the dried crystal product into a sealed capillary tube. Gently tap the tube to move the crystals down to the sealed end. 4. Place the tube into the melting point apparatus ensuring that you can see the crystals through the viewing window. 5. Watch the thermometer closely as the temperature rises and record accurately the temperature at which the solid crystals melt. 6. Repeat steps 3-5 twice more to ensure that you record an accurate melting point. Part 3- Testing the purity of Aspirin (To be completed in the University) 1. Take a few crystals of your prepared aspirin and add 1ml of ethanol. 2. Add 1 drop of 0.1M Ferric Chloride Solution (FeCl3). 3. Observe the colour change. Record your findings. 4. Test the melting point of the commercial aspirin sample provided. 5. Take a few crystals of commercial aspirin and add 1ml of ethanol. 6. Add 1 drop of 0.1M Ferric Chloride Solution (FeCl3). 7. Observe the colour change. Record your findings. Part 4- Purifying Aspirin (To be completed in school) 1. If your sample is shown to be impure, you need to purify it. 2. Take a few crystals of your prepared aspirin and add warm ethanol. 3. Allow to cool slowly, re-crystallising the aspirin 4. Set up a Buchner Funnel and insert filter paper. 5. Wet the filter paper with distilled water. 6. Filter the solid aspirin using the Buchner Funnel. 7. Wash the crystals using 3ml of chilled water. 8. Dry the crystals and filter paper using an oven overnight at 37°C. 9. Weigh the solid and filter paper and record your results. 10. Re-test the purity as detailed in Part 3. Questions 1. What is the actual weight of your synthesised aspirin? 2. What is the theoretical weight of aspirin produced? C7H6O3 + C4H6O3 H3PO4 C9H8O4 + Salicylic acid + Acetic anhydride C2H4O2 Aspirin + Acetic Acid In a 1:1 reaction the number of moles of salicylic acid used = number of moles of aspirin produced. Moles of salicylic acid used in this reaction = Grams of salicylic acid used RMM of 1 mole of salicylic acid = moles Theoretical Moles of aspirin produced Theoretical mass of aspirin = moles = (Number of moles used) x (RMM of 1 mole of aspirin) = grams 3. Calculate the percentage (%) yield of you synthesised aspirin % Yield = Actual Yield (g) x100% Theoretical Yield (g) 4. Is the % yield more or less than 100%? Explain your results. 5. Ferric Chloride solution was added to your synthesised aspirin in part 3 to produce a coloured reaction. Describe the difference in colour of your synthesised aspirin reaction to the sample of shop bought aspirin reaction. 6. Does the melting point of your synthesised aspirin match that of the shop bought aspirin? Explain any differences. Technicians Notes (Per group of students) Equipment 250ml Conical flask Heating mantle Filter paper Weighing balance Ice-bath Glass rod Buchner funnel Oven Melting point apparatus Test-tube Chemicals 3g Salicylic Acid 6ml Acetic anhydride 5-10 drops phosphoric acid (85%) 25ml distilled water 2ml Ethanol GPR 2 drops FeCl3 0.1M