APP_38027_sm_SuppInfo
advertisement

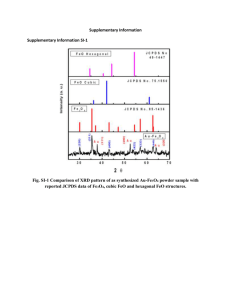
Supporting Information Magnetite-Embedded Electrospun Polyacrylonitrile-Based Carbon Fibers: Synthesis and Electromagnetic Wave Absorption Characteristics Ying Yang1,2 Zhen Guo3 Huan Zhang1 Daqing Huang3 Jialin Gu3 Zhenghong Huang3 Feiyu Kang3 T Alan Hatton1 Gregory C Rutledge1 1 Department of Chemical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA) 2 Department of Electrical Engineering, Tsinghua University, Beijing, 100084, China. 3 Department of Materials Science and Engineering, Tsinghua University, Beijing,100084 China A. Solution Rheology. Composite solutions prepared with Fe3O4 nanoparticles exhibit higher zero-shear-rate viscosity and sharper shear thinning behavior than do pure PAN solutions. Shear deformation is the most common mode of polymer deformation, but stretching flow occurs in fiber spinning, blow molding, film blowing, injection molding, and coating of films. So, extensional deformation [1] came to the fore in the last two to three decades. Extensional viscosity is the measure of the resistance of a material subjected to stretching flow and is identified by the extensional stress measured in the test divided by the constant strain rate. Fig. S1(a) shows shear viscosity measured in a Thermo Scientific HAAKE VT550 Viscometer viscometer for several solutions of PAN in DMF with various amounts of Fe3O4 added. Fig S1(b) shows the variation with time of the filament diameter in the CaBER extensional rheometer for several solutions of PAN in DMF with various amounts of Fe3O4 added. The addition of iron oxide serves to increase both the shear viscosity and the relaxation time (obtained from the inverse of the slope of diameter vs time in Fig S1(b)) of the PAN/DMF solution. (a) (b) Fig. S1 Rheological behavior (a)viscosity vs. shear rate; and (b) extensional viscosity B. Magnetic hysteresis loops Fig. S2 Magnetic hysteresis loops at different particle loadings in Fe3O4/PAN composite fibers at room temperature. Inset shows linear correlation of saturation magnetization with Fe3O4 loading. C. Calculation of concentration of magnetic nanoparticles in carbon fiber For purpose of this calculation, we assume that all of the nanoparticles were Fe3O4. If the Fe3O4 were reduced to Fe or Fe3C, the weight % would be smaller than that calculated under this assumption, so that the estimates calculated here serve as upper bounds. Based on the TGA results in Figure S3, it can be seen that around 30% of the weight was left after heating to 800°C. The actual weight loss for each sample upon heating to 800°C is presented in Table S1. Fig. S3. The degradation trends in nitrogen environments for as-electrospun Fe3O4/PAN fibers by thermogravimetic analysis Thermogravimetric analysis (TGA) was conducted using a Q5000IR thermogravimetric analyzer (TA Instruments, Inc.). Samples were subjected to heating scans (3°C/min) in a temperature ramp mode.. Table S1 The degradation trends in nitrogen environments for as-electrospun Fe3O4/PAN fibers by TGA Fe3O4 loading in PAN 0% 2% 7% 17% Weight lost 70% 70% 83% 64% Based on this information, the content of iron in the form of iron oxide in each sample of carbon fibers was estimated as follows. (1) For 1 wt % sample (nominally 2% Fe3O4/C fibers); 1 g 2% Fe3O4/PAN fibers produces ~0.3g nominally 2% Fe3O4/C fibers The real concentration Fe3O4 in carbon fiber should then be 0.02g/0.3g=6.7 wt% The concentration of Fe3O4 in EM absorber is then 1wt%*6.7wt%=0.067wt% (2) For 5 wt % sample (nominally 2% Fe3O4/C fibers) 1 g 2% Fe3O4/PAN fibers produces ~0.3g nominally 2% Fe3O4/C fibers The concentration of Fe3O4 in EM absorber should then be 5wt%*6.7wt%=0.33 wt% (3) For 5 wt % sample (nominally 17% Fe3O4/C fibers) 1 g 17% Fe3O4/PAN fibers produces ~0.36g nominally 17% Fe3O4/C fibers The real concentration Fe3O4 in carbon fiber should then be 0.17/0.36=0.47 wt% The concentration of Fe3O4 in EM absorber should then be 5%*47%=2.4 wt% References: [1] Chen L, Bromberg L, Hatton TA, and Rutledge GC. Electrospun cellulose acetate fibers containing chlorhexidine as a bactericide. Polymer 2008;49(5):1266-1275