Guidance for individuals associated with the (UL1)
advertisement

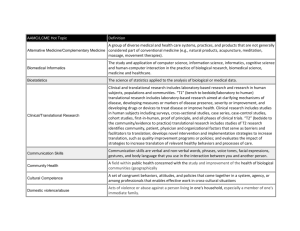
CTSI Suggested Format for Biosketch and Other Support Pages Program Year 8 – 7/01/2013 through 6/30/2014 Biosketch Section C – Suggested Format 5 UL1 TR000042-08 (P.I. Kieburtz, Karl D.) 7/01/2011 - 6/30/2016 5 KL2 TR000095-08 (P.I. Kieburtz, Karl D.) 5 TL1 TR000096-08 (P.I. Kieburtz, Karl D.) NIH/National Center for Research Resources and National Center for Advancing Translational Sciences The University of Rochester’s Clinical and Translational Science Institute The major goal of this project is to make the University of Rochester Clinical and Translational Science Institute an academic home for clinical and translational sciences, providing a centralized, integrated infrastructure. Role: [role within key function, name of key function] NOTES: 1. The percent effort and amount of funding (direct dollars) are not noted on Biosketches, only on Other Support pages. 2. The section containing the information on the UL1 grant, including major goals, should be copied verbatim. 3. The section describing the role of the individual must be customized based on his or her role on the grant. Many individuals will have roles on more than one key function, so repeat this section as necessary. 4. Key function directors should describe their role as Principal Investigator or Co-Principal Investigator, as appropriate. 5. Key function names are provided in the table below. Key Function Approach and Governance Pilot Translational and Clinical Studies Incubator Program Biomedical Informatics Regulatory Knowledge and Support and Clinical Research Ethics Clinical Research Resources and Facilities Community Engagement and Research Research Education, Training and Career Development Upstate New York Translational Research Network (UNYTE) Evaluation Comparative Effectiveness Research Public Private Partnerships T1 Translations Administrative Supplement The University of Rochester Clinical and Translational Science Institute Ltd Comp: Rev Apps to Advance Evidence-Based Research Related to Protections for Humans Subjects 106747605 Page 1 of 3 CTSI Suggested Format for Biosketch and Other Support Pages Program Year 8 – 7/01/2013 through 6/30/2014 Other Support Pages - Suggested Format 5 UL1 TR000042-08 (P.I. Kieburtz, Karl D.) 7/01/2011 - 6/30/2016 5 KL2 TR000095-08 (P.I. Kieburtz, Karl D.) 5 TL1 TR000096-08 (P.I. Kieburtz, Karl D.) NIH/National Center for Research Resources and National Center for Advancing Translational Sciences $3,198,387 The University of Rochester’s Clinical and Translational Science Institute The major goal of this project is to create the University of Rochester Clinical and Translational Sciences Institute as the academic home for clinical and translational sciences, providing a centralized, integrated infrastructure. Role: [role within key function, name of key function] xx.x person-months (cal) xx% FTE $xxx,xxx Notes: 1. The section containing the information on the UL1 grant, including major goals, should be copied verbatim. The annual direct costs of the program will be updated each program year. 2. The section describing the role of the individual must be customized based on his or her role on the grant. Many individuals will have roles on more than one key function, so repeat this section as necessary. 3. Key function directors should describe their role as Principal Investigator or Co-Principal Investigator, as appropriate. 4. Key function names are provided in the table below. The dollar figure is the annual direct costs related to the particular key function, as provided below. These are Year 8 figures; they will be updated each year. Key Function Approach and Governance Pilot Translational and Clinical Studies Incubator Program Biomedical Informatics Regulatory Knowledge and Support and Clinical Research Ethics Clinical Research Resources and Facilities Community Engagement and Research Research Education, Training and Career Development Upstate New York Translational Research Network (UNYTE) Evaluation Public Private Partnerships T1 Translations Comparative Effectiveness Research TL1 Training Program Mentored Career Development Award KL2 Scholar Program 106747605 Annual Direct Costs ($) 357,344 290,337 256,745 334,702 394,980 257,353 417,120 100,652 52,331 11,948 11,999 11,948 216,160 389,014 Page 2 of 3 CTSI Suggested Format for Biosketch and Other Support Pages Program Year 8 – 7/01/2013 through 6/30/2014 Administrative Supplement The University of Rochester Clinical and Translational Science Institute Ltd Comp: Rev Apps to Advance Evidence-Based Research Related to Protections for Humans Subjects 106747605 Annual Direct Costs ($) 95,755 Page 3 of 3