6th Grade Science: Elements and Compounds Lesson Plan
advertisement

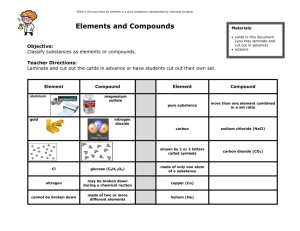
Lesson Plan Teacher: Bonnie Sharp Date(s): September 13-16 Subject area / course / grade level: Science/ 6th grade Materials: Scissors glue elements sheet periodic table TEKS/SEs: SCI.6.1A Demonstrate safe practices during laboratory investigations as outlined in the Texas Safety Standards SCI.6.2C Collect and record data using the International System of Units (SI) and qualitative means such as labeled drawings, writing and graphic organizers. SCI.6.4A Use appropriate tools to collect, record and analyze information SCI.6.5A Know that an element is a pure substance represented by chemical symbols. SCI.6.5C Differentiate between elements and compounds on the most basic level. SCI.6.6A Compare metals, nonmetals and metalloids using physical properties such as luster, conductivity or malleability. ELPS: ELPS.c.1C , ELPS.c.1E Lesson objective(s): We will identify that elements combine to make compounds. I will work with my partner to use TAILS to make an excellent graph. Instructional strategies: COMBINING ELEMENTS: Determine elements found in listed compounds. Write the symbol or formula for each element. Graph data. Differentiation strategies to meet diverse learner needs: Hands On Demonstration Classroom Discussion Visual Examples Evaluation of student learning: Exit Ticket 1 Lesson Plan Teacher: Bonnie Sharp Date(s): September 17-18 Planning Day WEDNESDAY Subject area / course / grade level: Science/ 6th grade Materials: Periodic Table Element/Compound Cards TEKS/SEs: SCI.6.1A Demonstrate safe practices during laboratory investigations as outlined in the Texas Safety Standards SCI.6.2C Collect and record data using the International System of Units (SI) and qualitative means such as labeled drawings, writing and graphic organizers. SCI.6.4A Use appropriate tools to collect, record and analyze information SCI.6.5A Know that an element is a pure substance represented by chemical symbols. SCI.6.5C Differentiate between elements and compounds on the most basic level. SCI.6.6A Compare metals, nonmetals and metalloids using physical properties such as luster, conductivity or malleability. ELPS: ELPS.c.1C , ELPS.c.1E Lesson objective(s): We will use my knowledge of elements and compounds to write the chemical formulas of compounds. I will work with my partner to determine is a substance is an element or a compound Instructional strategies: The Difference Between Elements and Compounds You have learned how to write the symbols for the elements. You should also remember that elements are pure substances while compounds are not. You have also learned to write symbols for a compound, which is called a formula. Remember that if there is only one element in the compound there is no subscript written. If there is more than one atom in the molecule a subscript number is placed after the element in the formula. Directions: In the activity below you will write the chemical formula for each compound using the element symbols and the subscript numbers. Use the periodic table. 1. Rust is also called iron oxide. It has two parts iron and three parts oxygen. Write the formula for iron oxide _________________ How many elements are in this compound? _____ 2. Baking soda has one part sodium, one part hydrogen, one part carbon and three parts oxygen. Write the formula for baking soda ________________ How many elements make up this compound?_____ 2 Lesson Plan 3. Glucose is a type of sugar we find in plants. It has six parts carbon, twelve parts hydrogen and six parts oxygen. Write the formula for glucose ___________________ How many elements are in this compound? _____ 4. Carbon monoxide is a very deadly gas that is made by cars. It has one part carbon and one part oxygen. Write the formula for carbon monoxide __________ How many elements are in this compound?_____ 5. Methane is a gas that is made in the human body. It has one part carbon and four parts hydrogen. Write the formula for methane _________________ How many elements are in this compound?_____ 6. Sand is made up of tiny crystals of silicon dioxide. It has one part silicon and two parts oxygen. Write the formula for silicon dioxide _____________ How many elements are in this compound? ______ 7. Hydrogen peroxide is used to clean cuts without stinging. It has two parts hydrogen and two parts oxygen. Write the formula for hydrogen peroxide _________ How many elements are in this compound? ______ Questions: 1. How many elements of carbon are in CO2? __________ 2. How do you know? ___________________________ 3. How many elements make up CO2? ______________ 4. How do you know? ___________________________ Look at the cards in the basket with your partner. Determine if the substance on each card is an element or a compound. Write the information in the chart below. Name Copy the Symbol or Is it an Element Is it a pure Formula or a Compound substance? (Yes or No) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 3 Lesson Plan Questions: 1. How did you determine if the substance on the card was an element or a compound? 2. Which substances are pure substances? 3. Which substances are not pure substances? Differentiation strategies to meet diverse learner needs: Classroom Discussion Visual Examples Graphic Organizer Evaluation of student learning: Exit Ticket 4 Lesson Plan Teacher: Bonnie Sharp Date(s): September 19-20 Lab Day FRIDAY Subject area / course / grade level: Science/ 6th grade Materials: Teacher Demo baking soda, Damp Rid 2 -250 mL beakers with 50 mL of water small spoon TEKS/SEs: SCI.6.1A Demonstrate safe practices during laboratory investigations as outlined in the Texas Safety Standards SCI.6.2C Collect and record data using the International System of Units (SI) and qualitative means such as labeled drawings, writing and graphic organizers. SCI.6.4A Use appropriate tools to collect, record and analyze information SCI.6.5A Know that an element is a pure substance represented by chemical symbols. SCI.6.5C Differentiate between elements and compounds on the most basic level. SCI.6.6A Compare metals, nonmetals and metalloids using physical properties such as luster, conductivity or malleability. ELPS: ELPS.c.1C , ELPS.c.1E Lesson objective(s): We will classify substances as a pure substance or a mixture (solution). I will work with a partner to complete a concept map that classifies matter. Instructional strategies: Learning About Pure Substances Scientists often examine the particles that make up a substance. Close observation shows that in some cases all the particles that make up a substance are alike. In other cases all the particles are not alike. When all the particles are alike the substance is called a pure substance. A pure substance is made of only one kind of substance and is the same throughout. All the particles in a pure substance are exactly the same. Elements are the simplest pure substance. They cannot be changed into a simpler substance by any chemical or physical process. Compounds are also pure substances. Water, salt and sugar are compounds that are pure substances because even though they are made of more than one element they are the same throughout. So, compounds are pure substances and cannot be broken down into simpler substances without changing their properties. Elements cannot be broken down into simpler substances without losing their identity. If you place a pure substance into water you create a solution. A solution is a mixture – not a pure substance. Kool-Aid and lemonade are solutions. 5 Lesson Plan Teacher Demo Materials: baking soda, Damp Rid, 2 -250 mL beakers with 50 mL of water, small spoon What To Do: 1. Observe the baking soda your teacher shows you. It is a compound with the chemical formula NaHCO3. Is it the same through out? ______ Is it a pure substance? _____ 2. Observe the calcium chloride your teacher shows you. It is a compound with the chemical formula CaCl2. Is it the same through our _____ Is it a pure substance? ____ 3. Observe the beakers of water your teacher shows you. It is the same throughout? ____ Is it a pure substance? ______ 4. Your teacher will now mix half a small spoon of baking soda in one of the beakers of water until it is clear. It is the same throughout? ____ Is it a pure substance? ______ 5. Your teacher will now mix half a small spoon of Damp Rid in the other beaker of water. It is the same throughout? ____ Is it a pure substance? ___ 6. Your teacher will now pour the solution of baking soda into the solution of Damp Rid. Observe what happens. Observations: 1. In the beakers below draw what happened in the demonstration. + Baking soda and water Calcium chloride and water Calcium Carbonate Salt and water When two liquids are mixed together, sometimes they form a solid. When this happen the solid is called a precipitate and it is a chemical change. In this demonstration the water became cloudy with the precipitate. Your teacher will keep it over night and you will observe it again tomorrow. Cut out the words in the table and glue them into the correct position on the concept map below. Fill in the blanks with examples we have used in this unit. 6 Lesson Plan Differentiation strategies to meet diverse learner needs: Hands On Demonstration Classroom Discussion Visual Examples Graphic Organizer Evaluation of student learning: Exit Pass 7