Does this proposed regulation conform to the relevant international
advertisement

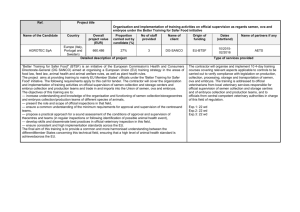
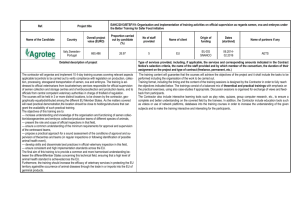
WORLD TRADE G/SPS/N/EEC/380 28 July 2010 ORGANIZATION (10-4040) Committee on Sanitary and Phytosanitary Measures Original: English NOTIFICATION 1. Notifying Member: EUROPEAN UNION If applicable, name of local government involved: 2. Agency responsible: Commission of the European Communities. Health & Consumers Directorate-General Directorate D - Animal Health and Welfare 3. Products covered (provide tariff item number(s) as specified in national schedules deposited with the WTO; ICS numbers should be provided in addition, where applicable): Semen of animal of the ovine and caprine species (CN 0511 99 85) 4. Regions or countries likely to be affected, to the extent relevant or practicable: [] All trading partners [X] Specific regions or countries: Australia, Canada, Switzerland, Chile, Greenland, Croatia, Iceland, New Zealand, Saint Pierre and Miquelon and United States 5. Title of the notified document: Draft Commission Decision of *** on imports of semen, ova and embryos of animals of the ovine and caprine species into the Union" Language: English Number of pages: 22 http://members.wto.org/crnattachments/2010/sps/EEC/10_2912_00_e.pdf 6. Description of content: The purpose of this Decision is to align accordingly the model health certificates for imports into the Union of ova, embryos and semen of animals of the ovine and caprine species taking into account the amendments made to Directive 92/65/EEC by Directive 2008/73/EC and Regulation (EU) No 176/2010 and therefore to repeal Decision 2008/635/EC. 7. Objective and rationale: [ ] food safety, [X] animal health, [ ] plant protection, [ ] protect humans from animal/plant pest or disease, [ ] protect territory from other damage from pests. 8. Is there a relevant international standard? If so, identify the standard: [] Codex Alimentarius Commission (e.g. title or serial number of Codex standard or related text) [X] World Organization for Animal Health (OIE) (e.g. Terrestrial or Aquatic Animal Health Code, chapter number) Terrestrial Animal Health Code of the OIE [] International Plant Protection Convention (e.g. ISPM number) [] None Does this proposed regulation conform to the relevant international standard? [X] Yes [ ] No . /. G/SPS/N/EEC/380 Page 2 If no, describe, whenever possible, how and why it deviates from the international standard: 9. 10. Other relevant documents and language(s) in which these are available: "Commission Regulation (EU) No 176/2010 of 2 March 2010 amending Annex D to Council Directive 92/65/EEC as regards semen collection and storage centres, embryo collection and production teams, and conditions for donor animals of the equine, ovine and caprine species and for handling semen, ova and embryos of those species" (OJ L 52, 3 March 2010, p.14) http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:052:0014:0027:EN:PDF "Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A(I) to Directive 90/425/EEC (OJ L 268, 14 September 1992, p. 54), and in particular Article 17(2)(b), Article 17(3), the first indent of Article 18(1), and the introductory phrase and point (b) of Article 19 thereof" http://eurlex.europa.eu/Notice.do?val=186121:cs&lang=en&list=489159:cs,207326:cs,195780:c s,186121:cs,&pos=4&page=1&nbl=4&pgs=10&hwords Proposed date of adoption (dd/mm/yy): 15 August 2010 Proposed date of publication (dd/mm/yy): 20 August 2010 11. Proposed date of entry into force: [ ] Six months from date of publication, and/or (dd/mm/yy): 1 September 2010 [X] 12. Trade facilitating measure Final date for comments: [X] Sixty days from the date of circulation of the notification and/or (dd/mm/yy): 25 September 2010 Agency or authority designated to handle comments: [X] National Notification Authority, [X] National Enquiry Point. Address, fax number and e-mail address (if available) of other body: European Commission DG Health and Consumers, Unit D3 - International questions (multilateral) Rue Froissart 101 - B 1049 Brussels Tel.: +(32 2) 29 68185/59922 Fax: +(32 2) 29 98090 E-mail: sps@ec.europa.eu 13. Texts available from: [X] National Notification Authority, [X] National Enquiry Point. Address, fax number and e-mail address (if available) of other body: European Commission DG Health and Consumers, Unit D3 - International questions (multilateral) Rue Froissart 101 - B 1049 Brussels Tel.: +(32 2) 29 68185/59922 Fax: +(32 2) 29 98090 E-mail: sps@ec.europa.eu