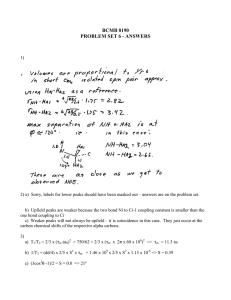
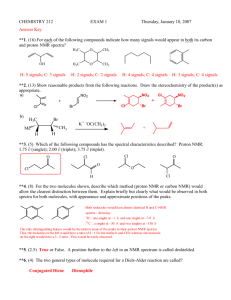
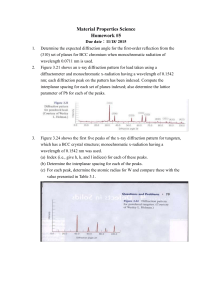
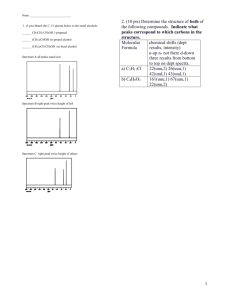
NMR study sheet
advertisement

Chapter 13 Nuclear Magnetic Resonance (NMR) Spectroscopy Recap: Mass Spectrometry – molecular formula and size Infrared spectroscopy – functional groups present or absent Ultraviolet spectroscopy – existence of a conjugated π-electron system and how many NMR spectroscopy – map of carbon-hydrogen framework (put the pieces together) For Organic chemistry, we’re interested in 1H and 13C NMR Use tetramethylsilane (TMS) as our standard and set its peak at 0.00 ppm or 0.00 δ. The position on the spectrum is called the chemical shift. Downfield is above 0.00 ppm or to the left-hand side; upfield is below 0.00 ppm (negative number) or to the right-hand side. 13 C NMR In general: sp3-hybridized C’s absorb from 0-90 δ; sp2-hybridized C’s absorb from 110-220 δ; sp-hybridized C’s absorb from 75-95 δ; carbonyl C’s (C=O) absorb from 160-220 δ. Broadband-decoupled: the H’s are also irradiated so that there is no spin-spin splitting patterns. Results are that there is 1 unique peak for each unique C in the structure. e.g. 1-bromobutane would show 4 peaks; 1-bromo-2-methylpropane would show 3 peaks. Distortionless Enhancement by Polarization Transfer (DEPT-NMR): A series of experiments are run such that the CH3, CH2, CH, and C carbons are distinquished from one another. In the DEPT-90 spectrum, the CH carbons are identified. In the DEPT-135 spectrum, the CH3 and CH carbons are displaced as positive peaks, and the CH2 carbons are displayed as negative peaks. The quaternary C’s (no H’s) are identified by noting which C’s are present in the broadband experiment but never appear in the DEPT experiments. 1 H NMR In general: H’s on sp3-hybridized C’s absorb from 0-1.5 δ; H’s on sp2-hybridized C’s absorb from 4.5-6.5 δ; H’s on sp-hybridized C’s absorb from 2.5-3.0 δ; H’s on aromatic rings absorb from 6.5-8.0 δ; H’s on carbons bonded to electronegative atoms or adjacent to carbons bonded to electronegative atoms will shift the absorbance downfield (higher number). Each unique type of hydrogen will have its unique chemical shift. However, the peaks are not often single peaks. Spin-spin splitting is seen due to the neighboring H’s. Use the “n + 1” rule to determine the splitting or coupling pattern. n = the number of H’s on the adjacent carbon or carbons. e.g. CH3 – CH2 – Br CH3 hydrogens are adjacent to a carbon with 2 H’s; thus the peak appears as a triplet (2+1) CH2 hydrogens are adjacent to a carbon with 3 H’s; thus the peak appears as a quartet (3+1) The total area of the peaks will be in the correct ratio (2:3); since this will be hard to determine by sight, the spectrum is usually “integrated” which means that the computer will draw a line that can be measured to indicate the ratios. (Computer spits out the number on a printout) # of adjacent Hydrogens Coupling or splitting pattern Ratio 0 1 peak, singlet (s) 1 1 2 peaks, doublet (d) 1:1 2 3 peaks, triplet (t) 1:2:1 3 4 peaks, quartet (q) 1:3:3:1 4 5 peaks, quintet 6 7 peaks, septet Hydrogens can show coupling from more than one set of H’s = complex pattern! Chemically equivalent protons do not show spin-spin splitting The signal of a proton that has n equivalent neighboring protons is split into a multiplet of n + 1 peaks with coupling constant J. Two groups of protons coupled to each other have the same coupling constant, J.