powerpoint
advertisement

Lecture 37
Nuclear magnetic resonance
Nuclear magnetic resonance
The use of NMR in chemical research
was pioneered by Herbert S.
Gutowski of Department of
Chemistry, University of Illinois, who
established the relationship between
chemical shifts and molecular
structures. He also discovered spinspin coupling.
Foundation of magnetic spectroscopy.
Proton NMR.
Circular electric current = magnet
Electrons in p, d, f orbitals
Electron spin
Nuclear spin
charge
magnetic
moment
q
m=
l
2m
mass
angular
momentum
Magnet-magnetic-field interaction
high energy
Classical
DE = - m × B
Quantum
Ĥ1 = - m̂ × B
low energy
q ˆ
=l ×B
2m
qB ˆ
=lz
2m
Tesla
C
J
T (Tesla)
qB
DE = lz
2m
kgm2/s
kg
1 T = 1 V s / m2
Nikola Tesla
Public domain image from Wikipedia
Field strength in 500 MHz NMR ($0.5M) = 11.7 T
Field strength in 1 GHz NMR ($20M) = 23.5 T
Strongest continuous magnetic field = 45 T
(National High Magnetic Field Lab at Tallahassee, FL)
Electrons in p, d, f orbitals
First-order perturbation theory
qB (0) ˆ (0)
qB
DE = Y 0 lz Y 0 = ml
2m
2m
e
Bohr magneton
=
ml B = mB ml B
−24
9.724×10 J/T
2me
qB ˆ
Ĥ 1 = lz
2m
ml = +2 ; DE = +2 mB B
(2 l + 1)-fold
degeneracy
(field off)
ml = +1 ; DE = +1mB B
ml = 0 ; DE = 0 mB B
ml = -1 ; DE = -1mB B
ml = -2 ; DE = -2 mB B
Zeeman effect (field on)
Electron spin
Quantum electrodynamics
DEorbit = mBml B
DEe-spin = ge mBms B
g-value
2.002319…
α
2-fold
degeneracy
(field off)
1
1
ms = + ; DE = + ge mB B
2
2
ge mB B
β
1
1
ms = - ; DE = - ge mB B
2
2
ESR or EPR (field on)
Nuclear magneton e
1800 times smaller
than Bohr magneton 2mp
Nuclear spin
DEe-spin = ge mBms B
DEn-spin = - gI mN ms B
Negative sign
positive nuclear charge
Nuclear g-factor
proton: 5.586
β
2-fold
degeneracy
(field off)
Proton
mass
1
1
ms = - ; DE = + gI mN B
2
2
gI mN B
α
1
1
ms = + ; DE = - gI mN B
2
2
NMR (field on)
Proton NMR
Sweep coils
β
gI mN B
α
Radio freq
Sample
Proton NMR spectra
(1) Overall intensity
(2) Groups of peaks
(3) Relative intensities of groups of peaks
(4) Pattern in each group (hyperfine structure)
Overall intensity
β
N b = Na exp ( -DE / kBT ) » Na (1- DE / kBT )
DE = gI mN B
α
Na
excess
α spins
Intensity of a NMR signal
~ energy of RF radiation absorbed / time
~ ΔE × number of excess α spins
~ B2 / T
Stronger magnet + lower temperature
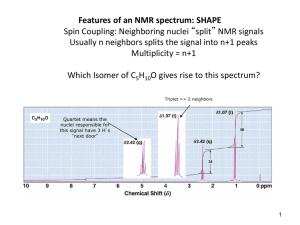
Group of peaks: chemical shifts
Resonance freq.
Chemical shift
β
DE = gI mN B
α
Resonance freq. of TMS
Si(CH3)4
n -n
6
d=
´10
n
“ppm”
Group of peaks: chemical shifts
Resonance freq.
Shielding
constant
n -n
6
d=
´10
n
hn = gI mN Blocal = gI mN (1- s ) B
Chemical shift
B
Blocal
Group of peaks: chemical shifts
hn = gI mN Blocal = gI mN (1- s ) B
B
+
Blocal
Shielding
constant
Group of peaks: chemical shifts
hn = gI mN Blocal = gI mN (1- s ) B
Shielding
constant
Group of peaks: chemical shifts
n -n
d=
´10 6
n
RCH3
-CH2-CHROH
ArOH
Ar-H
-CHO
-COOH
14
12
10
8
6
4
2
0
δ
Relative intensities
C2H6O
OH
H
H2
CH2
RCH3
-CH2-
H3
ROH
CH3
4
2
δ
CH3CH2OH
0
Hyperfine structure
Spin-spin coupling: hJms ms¢
H
β
CH3CH2OH
nearby H
1
- hJ
4
β
α
OH CH2
CH3
gI mN B
1
gI mN B - hJ
2
1
gI mN B + hJ
2
α
α
β
Hyperfine structure
Spin-spin coupling: hJms ms¢
H
CH3CH2OH
OH CH2
CH3
β
H2
H
β
α
gI mN B
α
α
β
ββ
βα, αβ
αα
αα
αβ, βα
ββ
Hyperfine structure
Pascal’s triangle
CH3CH2OH
1
nearby H
OH CH2
1
1
CH3
nearby H2
1
nearby H3
nearby H4
1
1
2
3
4
1
3
6
1
4
1
Hyperfine structure
CH3CH2OH
OH CH2
?
CH3
Q: Why doesn’t the proton in the
OH group cause splitting?
A: The proton undergoes a rapid
exchange with protons in other
ethanol or water molecules; its spin
is indeterminate in the time scale of
spectroscopic transitions; this
causes lifetime broadening of
spectral line rather than splitting.
Hyperfine structure
CH3CH2OH
OH CH2
?
CH3
?
Q: Why is there no spin-spin
coupling between the two protons
in the CH2 group?
A: There is spin-spin coupling
between them; however, its effect
on the peaks is null and
undetectable; this is because these
protons are chemically and
magnetically equivalent.
Hyperfine structure
CH3CH2OH
ì
a (1) a ( 2 )
ï
Triplet ï a (1) b ( 2 ) + b (1) a ( 2 )
í
magnetic
ï
b (1) b ( 2 )
ïî
Singlet
a (1) b ( 2 ) - b (1)a ( 2 )
non-magnetic
MS = 1
MS = 0
S =1
M S = -1
MS = 0
S=0
DEspin-orbit = 12 hcA { j ( j +1) - l ( l +1) - s ( s +1)}
DEspin-spin = 12 hJ {S ( S +1) - s ( s +1) - s¢ ( s¢ +1)} = 12 hJ {S ( S +1) - 23 }
M S = -1
MS = 0
MS = 1
no spin-spin coupling
+ 14 hJ
+ 14 hJ
No change in spacing
+ 14 hJ
with spin-spin coupling
Spin-spin coupling constant
1
J HH
2
J HH
3
J HH
H
H
H
H
C
C
H
C
H
Spin-spin coupling constant
H
Fermi
contact
H
Fermi
contact
DE = hJ ( 12 ) ( - 12 ) < 0
1
J HH > 0
Covalent bond
singlet-coupling
higher energy??
Fermi contact
lower energy!
Spin-spin coupling constant
H
Fermi
contact
C
H
Hund
Fermi
contact
Covalent
bond
singlet
coupling
Covalent
bond
singlet
coupling
DE = hJ ( 12 ) ( 12 ) < 0
2
J HH < 0
Spin-spin coupling constant
H
C
C
H
H
j
C
H
Karplus equation
3
J HH = A + Bcosj + C cos2j
Martin Karplus
Department of Chemistry
University of Illinois
Image (c) University of Illinois
ILLIAC
Magnetic resonance imaging: MRI
Resonance frequency
~ location (x)
x
Intensity
~ number of protons (in water) at x
Public domain image from Wikipedia
Paul Lauterbur (far right)
Department of Chemistry
University of Illinois
Summary
We have studied the foundation of magnetic
interactions and magnetic spectroscopy.
We have learned the theory of proton NMR
as an essential tool for chemical structural
analysis.
The origins of chemical shifts, hyperfine
structures, and spin-spin coupling constants
are discussed as well as their relation to
molecular structures.