Sample Exam #1 - Personal.kent.edu
advertisement

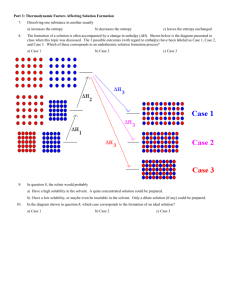
CHEM 10061 Exam #1 Name___________________ Date____________________ Cg1 Cg2 g ° Equations: V = nRT = MRT, n = m.w. , P = P , Pa = XaPa , T = m•K•i, g1 g2 Hv ap 1 P 1 q = m•s•T, 2d sin = n, ln 1 P2 R T2 T1 Constants: R = 0.0821 L-atm/mol-K or 8.314 J/mol-K or 62.36 L-torr/mol-K Multiple Choice (3 pts. each) ____1. Which of the following will be miscible with carbon tetrachloride, CCl 4? a. NH4Br b. Br2 c. CH3OH d. H2O Determine the value of i (van’t Hoff factor) for a dilute solution of LiBr. ____2. a. 1 b. 2 c. 3 d. 4 ____3. The exothermic step in the dissolution of an ionic compound in water is: a. b. c. d. separation of the ions in the salt separation of the water molecules hydration (solvation) of the ions in the salt separation of the ions in the salt and separation of the water molecules ____4. During osmosis a) b) c) d) ____5. pure solvent diffuses through a membrane but solutes do not. pure solutes diffuse through a membrane but solvents do not. pure solvent and a solution both diffuse at the same time through a membrane. gasses diffuse through a membrane into a solution and build up pressure. Which of the following is the most volatile? a. CCl4 b. CBr4 c. CF4 d. CH4 ____6. What is the molality of KBr in a solution made by dissolving 2.21 g of KBr in 897 g of water? a. 2.46 m b. 0.0167 m c. 0.0207 m d. 2.07 x 10–5 m ____7. As the concentration of a solute in a solution increases, the freezing point of the solution _________ and the boiling point of the solution __________. a. increases, increases c. decreases, increases b. increases, decreases d. decreases, decreases ____8. What is the molarity of 0.782 N sulfuric acid (H2SO4) solution? a. 0.782 M b. 0.391 M c. 0.261 M d. 1.56 M ____9. The vapor pressure of pure water at 25°C is 23.8 torr. Determine the vapor pressure of a solution prepared by dissolving 18.0 g of glucose (a non-volatile, non-electrolyte, C6H12O6) into 95.0 g of water at 25°C. a. 24.3 torr b. 23.4 torr c. 0.451 torr d. 0.443 torr ____10. The temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure is called the: a) heat of fusion c) boiling point b) heat of vaporization d) melting point ____11. The direct conversion of a solid to a gas is called: a. fusion b. vaporization c. condensation d. sublimation ____12. What is the concentration of magnesium ion in ppm for a 0.025 (w/w)% solution of MgCl2? a. 250 ppm b. 64 ppm c. 186 ppm d. 500 ppm ____13. Decreasing the total pressure above a liquid will cause the boiling point of the liquid to: a) increase c) remain the same b) decrease d) depends on the liquid ____14. Which solution will have the lowest freezing point? a. 0.030 m glucose(aq) ( a non-electrolyte) c. 0.030 m AlBr3(aq) b. 0.030 m CaBr2(aq) d. 0.030 m NaBr(aq) ____15. Identify the following unit cell: a) monoclinic c) primitive cubic b) body-centered cubic d) face-centered cubic ____16. Molality is defined as: moles solute a. moles solvent moles solute b. Liters solution moles solute c. kg solution d. ____17. Vapor pressure of a liquid is dependent upon: a) temperature c) volume and temperature b) volume d) depends on the liquid moles solute kg solvent ____18. Which one of the following will exhibit hydrogen bonding properties? a. CH3F b. F2 c. CH3NH2 d. H2S ____19. Calculate the mole fraction of urea (MW = 60 g/mol) in a solution prepared by dissolving 16 g of urea in 39 g of H2O. a. 0.36 b. 0.27 c. 0.89 d. 0.11 ____20. Which of the following would have the lowest vapor pressure at room temperature? a. CH3OH b. CH4 c. NH3 d. Br2 Solve the following problems showing all work. Use complete sentences for essay questions. Remember to use proper units, significant figures and rounding. 1. A solution was prepared by dissolving 6.00 g of a non-electrolyte into enough benzene to make 75.0 mL of solution. The density of the solution was determined to be 0.886 g/mL. The normal melting point of benzene is 5.50°C and the molal freezing point depression constant, Kf, for benzene is 5.12°C/m. The freezing point of the solution was determined to be 3.85°C. Determine the molecular weight of this substance. (10 pts.) 2. Bromine melts at -7.00°C and boils at 59.0°C. For this element the heat of fusion (Hfus) is 10.54 kJ/mol and the heat of vaporization (Hvap) is 30.0 kJ/mol. The specific heat of liquid bromine is 75.7 J/mol°C. Determine how much heat is required to convert 5.75 g of solid bromine at -7.00°C to liquid bromine at 30.0°C. (10 pts.) (Hint: Draw a heating curve for this transformation) 3. Draw a phase diagram for water and label all components of the diagram including triple point, critical point, axes, phase changes. (10 pts.) ? A: ? B: ? ??? C: ? ? ??? 4. List the following substances in order of increasing boiling point. answer. (10 pts.) HF, KBr, F2, Ne, CH3OH Justify your