Duplex and Color Doppler Sonography of the Liver - e
advertisement

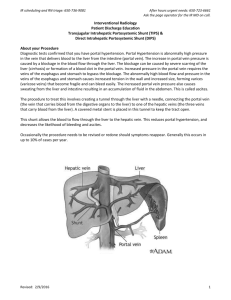
Duplex and Color Doppler Sonography of the Liver Author: Sharlene A. Teefey, M.D. Objectives: Upon the completion of this CME article, the reader will be able to: 1. List the etiologies of portal hypertension and describe the Doppler sonographic findings associated with this disorder. 2. Describe the collateral vessels that may be seen sonographically in patients with portal hypertension. 3. List the etiologies of portal vein thrombosis and describe the Doppler sonographic findings associated with this disorder. 4. List the etiologies of Budd-Chiari Syndrome and describe the Doppler sonographic findings associated with this disorder. Introduction The liver is the largest intra-abdominal organ in the human body and it is unique in that it has a dual blood supply. The two major avenues for blood entering the liver are through the hepatic artery and the portal vein. On average, approximately two fifths of the blood flow into the liver is from the hepatic artery and three fifths is from the portal vein. The portal vein is the major venous return from the intestines (therefore, the venous blood from the intestines must pass through the liver before it enters the rest of the systemic circulation). Upon entering the liver, both the hepatic artery and the portal vein branch into smaller vessels that eventually become capillaries. As the blood begins to exit the liver, these capillaries become venules and eventually form the hepatic veins that enter the inferior vena cava with blood flow back to the heart. In most circumstances, there are three main hepatic veins, the left, middle and right. Liver Doppler Technique Liver Doppler should be performed using a sector or curved array transducer with a frequency ranging between 2 to 6 MHz, depending on the patient’s body habitus. The scan should also be performed after an overnight fast to limit the amount of bowel gas in the region of the porta hepatis. When evaluating the portal venous system, the main portal vein as well as the right and left portal veins and their respective branches should be evaluated for patency and flow direction. The right and main portal veins are imaged best through an intercostal approach with the patient turned slightly to the left. The transducer should be angled obliquely and placed slightly laterally along the axis of a lower rib. An attempt should also be made to visualize the right and main portal veins from a subcostal approach, but frequently, this approach cannot be used due to overlying bowel gas. Because the left portal vein runs in an anterior direction, it is best visualized with the patient supine and with deep inspiration. The probe should be oriented in a parasagittal plane in the sub-xiphoid region of the patient. The hepatic venous system is best visualized with the patient supine or turned slightly to the left (at end-expiration if possible), especially if Doppler waveforms are to be obtained. Because of the superior location of the hepatic veins, deep inspiration may be necessary to improve visualization when using color Doppler. The left hepatic vein can be visualized with the transducer in a sub-xiphoid location either in a longitudinal or transverse plane. Transverse subcostal or slightly oblique images obtained more laterally or through an intercostal space will be useful when visualizing the middle and right hepatic veins. When visualizing the hepatic venous system, an effort should be made to show the communication of the hepatic veins with the inferior vena cava, especially in patients in whom the diagnosis of Budd-Chiari syndrome is suspected. Portal Hypertension Pressure within the portal venous system is the product of flow through the portal vein and the resistance to flow through the portal vein. The causes of portal hypertension can therefore be categorized based on these two factors. The primary etiology that causes an increase in flow is an arterio-portal venous fistula (which may be congenital, post-traumatic, atherosclerotic, or due to erosion of a hepatic artery aneurysm into the portal vein). The better-known category of primary increased resistance is traditionally divided into prehepatic, intrahepatic, and posthepatic causes. Prehepatic causes include portal or splenic vein thrombosis and extrinsic compression of the portal vein. Posthepatic causes of portal hypertension include constrictive pericarditis, inferior vena cava obstruction, and severe tricuspid insufficiency or right heart failure. Intrahepatic causes are based on the location of the increased resistance, which are perisinusoidal, sinusoidal, or post sinusoidal. Perisinusoidal causes of portal hypertension include parasitic infections (such as schistosomiasis), sarcoidosis, myeloproliferative diseases, early primary biliary cirrhosis, early primary sclerosing cholangitis, and toxins. Sinusoidal causes include alcoholic hepatitis or cirrhosis, and cryptogenic cirrhosis (cause unknown). Post sinusoidal causes include veno-occlusive disease and hepatic vein obstruction. Portal hypertension is also a frequent consequence of cirrhosis. In the setting of cirrhosis, factors that increase flow through the portal vein include vasoactive substances produced by the vascular endothelium such as nitric oxide and prostaglandins as well as the hormone glucagon. These substances cause splanchnic and systemic vasodilatation, which increases mesenteric and portal vein flow. Although these vasodilators are normally present in non-cirrhotic patients, it is theorized that there is an increased production of these vasodilators in the presence of cirrhosis or they are not cleared by the cirrhotic liver, resulting in elevated blood levels. Factors that increase resistance to portal vein flow are divided into fixed and variable components. Fixed components include fibrosis and regenerative nodules. Variable components include vasoconstrictors, such as endothelin, which is produced by endothelial cells and is a potent vasoconstrictor of smooth muscle, leading to increased portal vein resistance at the level of the sinusoids in the liver. Before discussing the sonographic findings in patients with portal hypertension, it is important to recall the gross and microscopic pathologic changes that can occur in patients with cirrhosis. Using alcoholic cirrhosis as a model, classic changes include hepatocyte necrosis, nodular regeneration, and the formation of fibrotic septae. However, there has been a more recent focus on hepatocyte enlargement secondary to alcohol-induced intracellular fat and protein deposition, which compresses the sinusoids and obstructs flow. Likewise, collagen deposition occurs in the space of Disse, which also increases resistance to portal venous flow. When resistance to flow increases beyond a pressure gradient of 10 to 12 mmHg, portosystemic collaterals develop that divert a portion of the portal venous flow. In an effort to compensate for the decreased flow in the portal venous system, hepatic artery flow increases. In about 10% of patients with severe end-stage liver disease, hepatic artery flow is diverted through the sinusoids to the portal vein producing hepatofugal flow (which is flow retrograde through the portal venous system away from the liver). There are several sonographic changes that can be detected in the portal vein, hepatic artery, and hepatic veins in patients with portal hypertension. Within the portal vein, flow may be hepatofugal (either within the right or left branch or the main portal vein); bidirectional (alternating between hepatopetal – flow toward the liver – and hepatofugal – flow away from the liver); or static, in which case flow cannot be detected with color Doppler sonography (figure 1). If no flow is seen on color Doppler, it is important not to misdiagnose this as portal vein thrombosis. The hepatic artery is often enlarged and tortuous with high peak systolic and diastolic velocities. This occurs in cases of advanced cirrhosis with decreased portal vein flow. There are also changes within the hepatic veins. Frequently, the waveform has a monophasic appearance with loss of the “a” wave, and low amplitude “D”/”S” waves. This is thought to be due to increased resistance to transmission of right atrial pressure changes secondary to focal hepatic vein stenoses. An important and specific sonographic finding in patients with portal hypertension is the presence of portosystemic collaterals. Collaterals may either be tributary collaterals (which are normally existing collaterals) or developed collaterals (which are normally closed channels that open because of the elevated pressure). The normally existing collaterals include the coronary or left gastric, short gastric, superior mesenteric, and inferior mesenteric veins (figure 2). Because of improvements in technology, the coronary vein can often be seen in normal patients, however, its diameter should be less than or equal to 6 mm and flow should be hepatopetal. In one recent study of patients with portal hypertension, the coronary vein was dilated in only 26% of patients. However, 78% of patients demonstrated hepatofugal flow and of those, 40% had a variceal bleed. Normally closed portosystemic collaterals that open in the setting of portal hypertension include the paraumbilical vein, the spleno-renal collaterals, and the splenoretroperitoneal collaterals. Again, due to the improvement in ultrasound equipment, flow may be demonstrated in a normal paraumbilical vessel; however, the flow is either very slow if hepatofugal (less than or equal to 5 cm per second) or hepatopetal and the vessel does not extend beyond the anterior liver surface. In patients with portal hypertension, the recanalized paraumbilical vein will extend beyond the anterior liver surface and show hepatofugal venous flow and a velocity greater than 5 cm per second. Portal Vein Thrombosis There are many causes of portal vein thrombosis including hypercoagulable states such as anti-phospholipid syndrome, myeloproliferative disease, birth control pill usage, and polycythemia vera. Inflammatory causes include inflammatory bowel disease, Behcet’s disease, and pancreatitis. Infectious diseases such as appendicitis and diverticulitis can also cause portal vein thrombosis. Medical interventions to treat neoplasms of the liver have also been shown to cause portal vein thrombosis such as chemo-embolization, alcohol injection, and partial hepatectomy. Portal vein thrombosis also occurs in patients undergoing liver transplant, sclerotherapy, and transjugular intrahepatic portosystemic shunting (TIPS). Miscellaneous causes include cirrhosis, hepatocellular carcinoma, and trauma. Sonographically, a portal vein thrombus may appear echogenic, isoechoic, or anechoic, and may be obstructive or non obstructive (figure 3). With color Doppler sonography, there will be no detectable flow. Recently, researchers described findings that distinguish benign from malignant portal vein thrombosis. The presence of a hepatofugal arterial signal within the thrombus had a high specificity for a malignant thrombus. However, its sensitivity was only 62%. Cavernous transformation is a sequela of portal vein thrombosis and implies the formation of porto-portal collaterals in the setting of an acquired, benign portal vein thrombosis (figure 4). It rarely occurs in patients with hepatocellular carcinoma. It can develop as early as 6 to 20 days after the thrombotic event. Collateral vessels and blood flow can originate from several sites including the paracholedochal veins, the periportal veins originating from around the pancreatic head, the vasovasorum of the portal vein wall, or recanalization of the portal vein itself. Doppler findings include non-visualization of the extrahepatic portal vein and or its branches with the formation of innumerable periportal collateral vessels. These vessels frequently extend intrahepatically around the thrombosed portal vein branches. Doppler analysis of these vessels shows a portal vein-like waveform. Patients with portal vein thrombosis and cavernous transformation not only develop portosystemic collaterals, (most commonly the left coronary vein or perisplenic veins), but also develop porto-portal collaterals. Reversed flow may develop in a patent portal vein branch to an area with portal vein thrombosis. Peri-cholecystic veins may also supply the posterior aspect of the right lobe of the liver. Budd-Chiari Syndrome Budd-Chiari Syndrome (thrombosis of the hepatic veins) has many different etiologies; however, recently, they have been classified into primary and secondary. Primary etiologies have further been divided into two categories, intrahepatic hepatic vein thrombosis and inferior vena cava thrombosis (hepatic portion). This latter category was previously referred to as membranous obstruction of the inferior vena cava. Recently, researchers have suggested that membranous obstruction of the inferior vena cava is an acquired rather than congenital phenomenon thought to be due to the formation of thrombus within the intrahepatic portion of the inferior vena cava. These two causes of Budd-Chiari Syndrome are distinctly different from one another. Thrombosis within the hepatic vein is common in developed Western countries, whereas inferior vena cava thrombosis is more common in underdeveloped countries such as Nepal, China, South Africa, and India. Common etiologies of intrahepatic hepatic vein thrombosis include hypercoagulable states such as anti-phospholipid syndrome, myeloproliferative diseases (which is thought to be the most common cause of Budd-Chiari Syndrome), protein C or protein S abnormalities, primary biliary cirrhosis, birth control pill usage, and collagen vascular diseases. Infection due to tuberculosis and schistosomiasis are also known to produce Budd-Chiari Syndrome. The etiology of inferior vena cava thrombosis, which might be due to hypercoagulable states in many cases, is still not fully understood. One recent theory proposes that the intima of the inferior vena cava may be damaged secondary to diaphragm movement from respiration. Secondary causes of Budd-Chiari Syndrome include compression or mechanical obstruction of the hepatic veins from a neoplasm (for example hepatocellular carcinoma, adrenal cancer, or renal cell carcinoma), an abscess, or hydatid cyst. Sonographically, a hepatic vein thrombus (like a portal vein thrombus) may be echogenic, isoechoic, or anechoic and obstructive or non-obstructive (figure 5). In fact, in many patients, the hepatic veins cannot be identified as discrete structures. With Doppler, there will either be no detectable flow within the hepatic vein, or reversed flow when a proximal obstruction is present. The waveform is often monophasic in this latter setting. Collaterals often develop between the hepatic veins, or between peripheral portal veins and hepatic veins, or between peripheral portal veins and sub-capsular veins in the setting of chronic Budd-Chiari Syndrome. Figures 1 Portal hypertension with slow hepatofugal flow in the main portal vein 2 Portal hypertension with coronary vein showing hepatofugal flow 3 Non-occlusive thrombus with porto-splenic confluence 4 Cavernous transformation of the main portal vein 5 Budd Chiari Syndrome with thrombosis of the right hepatic vein References or Suggested Reading: 1. Ralls PW. Color Doppler Sonography of the Hepatic Artery and Portal Venous System. Radiology 1990; 155: 517-525. 2. Lorenz J, Winsberg F. Focal Hepatic Vein Stenoses in Diffuse Liver Disease. JUM 1996; 15: 313-316. 3. Wachsberg RH, Simmons MA, Coronary Vein Diameter and Flow Direction in Patients with Portal Hypertension: Evaluation with Duplex Sonography and Correlation with Variceal Bleeding. AJR 1994; 162: 637-641. 4. Wachsberg RH, Obolevich AT. Blood Flow Characteristics of Vessels in the Ligamentum Teres Fissure at Color Doppler Sonography: Findings in Healthy Volunteers and in Patients with Portal Hypertension. AJR 1995; 164: 1403-1405 5. Pozniak M; Baus K. Hepatofugal Arterial Signal in the Main Portal Vein: An Indicator of Intravascular tumor Spreas. Radiology 1991; 180: 663-666. 6. Dodd GD; Memel DS, Baron RL, Eichner L, Santiguida LA. Portal Vein Thrombosis in Patients with Cirrhosis: Does Sonographic Detection of Intrathrombus Flow Allow Differentiation of Benign and Malignant Thrombus? AJR 1995; 165: 573-577. 7. DeGaetano AM, Lafortune M, Patriquin H, De Franco A, Aubin B, Paradis K. Cavernous Transformation of the Portal Vein: Paterns of Intrahepatic and Splanchnic Collateral Circulation Dertected with Doppler Sonography. AJR 1995; 165: 1151-1155. 8. Okuda K, Kage M, Shrestha SM. Proposal of a New Nomenclature for Budd-Chiari Syndrome: Hepatic Vein Thrombosis Versus Thrombosis of the Inferior Vena Cava at Its Hepatic Portion. Hepatology 1998; 28: 1191-1198. 9. Hosoki T; Kuroda C, Tokunaga K, Marukawa T, Masuike M, Kozuka T. Hepatic Venous Outflow Obstruction: Evaluation with Pulsed Duplex Sonography. Radiology 1989; 170: 733-737. 10. Kane R, Eustace S. Diagnosis of Budd-Chiari Syndrome: Comparison between Sonography and MR Angiography. Radiology 1995; 195: 117-121. 11. Lin EC. Peri-stent vascular channels after transjugular intrahepatic portosystemic shunt placement for Budd-Chiari syndrome: CT and US findings. Abdom Imaging 2001;26:191-3. 12. Killi RM. Doppler sonography of the native liver. Eur J Radiol 1999;32:21-35. 13. Chawla Y, Kumar S, Dhiman RK, et al. Duplex Doppler sonography in patients with Budd-Chiari syndrome. J Gastroenterol Hepatol 1999;14:904-7. 14. Naganuma H, Ishida H, Konno K, et al. Intrahepatic venous collaterals. Abdom Imaging 1998;23:166-71. About the Author Sharlene A. Teefey, M.D. is currently an Associate Professor of Radiology at the Mallinckrodt Institute of Radiology at Washington University School of Medicine in St. Louis Missouri. She is a member of numerous societies and organizations including the American College of Radiology, the Society of Radiologists in Ultrasound, and the American Institute of Ultrasound in Medicine. She is a reviewer of manuscripts for Radiology, the American Journal of Roentgenology, and Radiographics. She has more than 45 publications in peer review medical journals and has been a speaker at numerous institutions and conferences across the country. Examination: 1. Etiologies that cause a primary increased flow in the portal venous system include A. congenital arterio-portal venous fistula. B. erosion of a splenic artery aneurysm into the hepatic vein. C. post-traumatic arterio-portal venous fistula. D. A & B above E. A & C above 2. Primary increased resistance in the portal vein is traditionally divided into _____ causes. A. prehepatic B. intrahepatic C. D. E. posthepatic all of the above Only B & C above 3. Sinusoidal causes of portal hypertension include A. parasitic infections (such as schistosomiasis) B. sarcoidosis or myeloproliferative diseases C. alcoholic hepatitis or cirrhosis D. early primary sclerosing cholangitis E. hepatic vein obstruction 4. Portal hypertension is also a frequent consequence of cirrhosis. In the setting of cirrhosis, factors that increase flow through the portal vein include vasoactive substances produced by the vascular endothelium that cause splanchnic and systemic vasodilatation such as A. nitric oxide and prostaglandins B. glucagon C. endothelin D. all of the above E. only A & B above 5. Factors that increase resistance to portal vein flow are divided into fixed and variable components. Fixed components include A. fibrosis and regenerative nodules. B. vasoconstrictors C. substances such as endothelin D. all of the above. E. B & C above. 6. When resistance to flow in the portal venous system increases beyond a pressure gradient of _____, portosystemic collaterals develop, that divert a portion of the portal venous flow. A. 5 to 8 mmHg B. 10 to 12 mmHg C. 15 to 18 mmHg D. 18 to 21 mmHg E. 23 to 26 mmHg 7. There are several sonographic changes that can be detected within the portal vein in patients with portal hypertension. Which of the following statements is (are) true? A. flow may be hepatofugal, but only in the right branch of the vein. B. flow will not be bi- directional, it will either be hepatopetal or hepatofugal. C. flow may be static, in which case flow cannot be detected with color Doppler sonography. D. All of the above. E. Only A & B above. 8. On Doppler sonography in patients with portal hypertension, the hepatic artery is A. B. C. D. E. often enlarged but fairly straight with low systolic and diastolic velocities. often enlarged and tortuous with high peak systolic and diastolic velocities. often small but tortuous with low systolic and diastolic velocities. often small and fairly straight with high peak systolic and diastolic velocities. None of the above. 9. In patients with portal hypertension, there are also changes within the hepatic veins. Frequently, the Doppler waveform has A. a biphasic appearance with loss of the “a” wave, but high amplitude “D”/”S” waves. B. a biphasic appearance with the presence of the “a” wave, and low amplitude “D”/”S” waves. C. a monophasic appearance with loss of the “a” wave, but high amplitude “D”/”S” waves. D. a monophasic appearance with loss of the “a” wave, and low amplitude “D”/”S” waves. E. a biphasic appearance with the presence of the “a” wave, and “D”/”S” waves with a variable amplitude. 10. An important and specific sonographic finding in patients with portal hypertension is the presence of portosystemic collaterals. The normally existing collaterals include the A. coronary or left gastric vein B. superior mesenteric and inferior mesenteric veins C. paraumbilical vein D. all of the above E. Only A & B above 11. Because of improvements in technology, the coronary vein can often be seen in normal patients, however, its diameter should be A. less than or equal to 6 mm and flow should be hepatopetal. B. less than or equal to 6 mm and flow should be hepatofugal. C. less than or equal to 10 mm and flow should be hepatopetal. D. less than or equal to 10 mm and flow should be hepatofugal. E. less than or equal to 14 mm and flow should be hepatopetal. 12. Normally closed portosystemic collaterals that open in the setting of portal hypertension include the A. coronary or left gastric vein B. paraumbilical vein and the spleno-renal collaterals C. short gastric vein D. all of the above E. Only A & C above 13. Due to the improvement in ultrasound equipment, flow may be demonstrated in a normal paraumbilical vessel; however, the flow is A. very slow if hepatofugal (less than or equal to 5 cm per second). B. hepatofugal and the vessel does extend beyond the anterior liver surface. C. D. E. very fast if hepatofugal (greater than 15 cm per second). hepatopetal and the vessel does extend beyond the anterior liver surface. hepatofugal and the vessel does not extend beyond the anterior liver surface. 14. There are many causes of portal vein thrombosis. Which of the following statements is (are) true? A. Hypercoagulable states that can lead to portal vein thrombosis include appendicitis and diverticulitis. B. Inflammatory causes include inflammatory bowel disease, Behcet’s disease, and pancreatitis. C. Infectious diseases such as birth control pill usage and polycythemia vera can cause portal vein thrombosis. D. Medical interventions to treat neoplasms of the liver have also not been shown to cause portal vein thrombosis E. None of the above. 15. Sonographically, a portal vein thrombus may appear A. echogenic B. isoechoic C. anechoic D. all of the above E. Only B & C above 16. Cavernous transformation is a sequela of portal vein thrombosis and implies the formation of porto-portal collaterals in the setting of an acquired, benign portal vein thrombosis. These collateral vessels and blood flow can originate from A. the paracholedochal veins B. the periportal veins C. the vasovasorum of the portal vein wall D. re-canalization of the portal vein itself E. all of the above 17. Doppler findings in portal vein thrombosis with cavernous transformation include A. visualization of the extrahepatic portal vein B. visualization of periportal collateral vessels that do not extend intrahepatically around the thrombosed portal vein branches C. a portal vein-like waveform in the collateral vessels identified. D. all of the above E. Only A & B above 18. Budd-Chiari Syndrome (thrombosis of the hepatic veins) has many different etiologies; however, recently, they have been classified into primary and secondary. Which of the following statements is (are) true regarding primary etiologies? A. They are further divided into two categories, intrahepatic hepatic vein thrombosis and inferior vena cava thrombosis (hepatic portion). B. Common etiologies for intrahepatic hepatic vein thrombosis include damage to the intima of the vessel secondary to diaphragm movement from respiration. C. D. E. The etiology of inferior vena cava thrombosis is fairly clear and includes hypercoagulable states, myeloproliferative diseases (which is thought to be the most common cause of Budd-Chiari Syndrome), and protein C or protein S abnormalities. All of the above. Only A & C above. 19. Secondary causes of Budd-Chiari Syndrome include A. damage to the intima of the vessel secondary to diaphragm movement from respiration. B. hypercoagulable states C. compression or mechanical obstruction of the hepatic veins from a neoplasm an abscess, or hydatid cyst. D. all of the above. E. Only A & B above. 20. Sonographically, in Budd-Chiari Syndrome, a hepatic vein thrombus may be A. echogenic B. isoechoic C. anechoic D. all of the above E. only A & B above