URIC ACID
advertisement

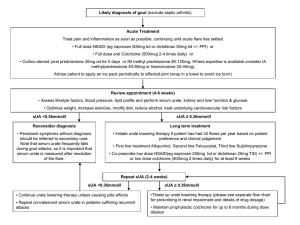
1 URIC ACID Dr Marita du Plessis Department of Chemical Pathology January 2002 INTRODUCTION: - Uric acid is the end product of purine metabolism. Hyperuricaemia is associated with a tendency to form crystals of monosodium urate causing: - Clinical gout (due to the deposition of monosodium urate crystals in the cartilage, synovium and synovial fluid of joints), - Renal calculi - Tophi (accretions of sodium urate in soft tissues) - Acute urate nephropathy (due to sudden increases in urate production leading to widespread crystallisation in the renal tubules). URIC ACID METABOLISM: (see Marshall, p 254-257) - - - Sources of purines in humans (see figure 1): - Diet - Degradation of endogenous nucleotides - De novo synthesis (energy requiring process). Purines are degraded to uric acid. Urate is excreted via 2 routes: - 1/3: Secretion into the gut, and subsequent degradation by bacterial uricase to CO2 and NH3. - 2/3: Renal excretion (see figure 2): - Urate is filtered at the glomeruli. - Proximal tubular reabsorption of 99% of filtered load. - More distal part of proximal tubules: secretion (also some reabsorption, but less than secretion). - Net excretion of 10% of filtered load. Body urate pool (and plasma concentration) depends on the relative rates of urate formation and urate excretion. Figure 1: Sources and excretion of urate Figure 2: Urate excretion in the kidney 2 Figure 3: Diagram of the pathways of purine nucleotide metabolism and uric acid synthesis in humans. APRT = adenine phosphoribosyl transferase; HGPRT = hypoxanthine-guanine phosphoribosyl transferase. - - - De novo synthesis leads to the formation of IMP (inosine monophosphate), which can be converted to AMP (adenosine monophosphate) and GMP (guanosine monophosphate) (NUCLEOTIDES: purine base + sugar + PO4). Nucleotide degradation involves the formation of the respective nucleosides (inosine, adenosine and guanosine) (NUCLEOSIDES: purine base + sugar), these are subsequently metabolised to the respective purine bases (hypoxanthine, adenine and guanine) (PURINE BASES). Hypoxanthine and guanine can be metabolised directly to xanthine, but AMP/adenosine have to be converted to IMP/inosine first. Xanthine is metabolised to uric acid by the enzyme xanthine oxidase, also responsible for conversion of hypoxanthine to xanthine. Because de novo synthesis is an energy requiring process, excretion of uric acid results in net energy loss. However, salvage pathways exist to convert purines back to their parent nucleotides and are therefore energy saving – accomplished by the following enzymes: - For guanine and hypoxanthine: HGPRT (hypoxanthine-guanine phosphoribosyl transferase). - For adenine: APRT (adenine phosphoribosyl transferase). 3 GOUT: - Gout is a group of metabolic diseases associated with hyperuricaemia and deposition of crystals of monosodium urate in tissues. Prevalence: 3/1000, males affected more than females (8-10:1). Presentation usually occurs in males over 30 years of age and females after the menopause. There are 4 stages in the development of the disorder: - 1. Asymptomatic hyperuricaemia: - Hyperuricaemia is usually present for many years before the onset of symptoms. - NB: Only 1 in 20 subjects with hyperuricaemia will eventually develop clinical gout. - 2. Acute gouty arthritis: - Classical presentation is acute inflammation of the metatarsophalangeal joint of the big toe (70%), and the first attack is usually monoarticular (affects only 1 joint). - Other joints that may be involved are the ankle, knee, wrist, elbow, and small joints of hands and feet. - 3. Intercritical gout: - Some patients may have only 1 attack, whilst others have recurrent attacks at shorter intervals. - Between attacks the patient is usually asymptomatic except for hyperuricaemia. - 4. Chronic tophaceous gout: - This follows recurrent attacks and is characterised by the development of tophi (swellings containing uric acid crystals) in the periarticular tissue. - Other sites include the helix of the ear, bursae and tendons. - Complications of hyperuricaemia: - Urolithiasis (kidney stones): - 10% of gouty patients develop urate stones and 10% of all renal calculi are due to urate. - Renal failure: - Acute renal failure due to obstructive uropathy (urate crystals) may occur during cytotoxic treatment of malignancy (allopurinol cover should be used), and has also been described in gouty subjects after severe exercise. - Progressive chronic renal insufficiency is an important cause of morbidity and mortality in untreated chronic tophaceous gout. - Associated conditions: - Alcoholism - Dysmetabolic syndrome (Insulin resistance syndrome)(syndrome X): Obesity, characteristic dyslipidaemia (increased triglycerides, decreased HDL cholesterol, small dense LDL), hypertension, impaired glucose tolerance, prothrombotic state. - Diagnosis: The laboratory evaluation of hyperuricaemia is discussed below. It is important to recognise that: - Hyperuricaemia is not synonymous with gout (1 in 20 develop gout) - Gout can be precipitated by a sudden change (either increase or decrease) in urate concentration. - An acute gout attack may be associated with a normal plasma urate level (due to a fall in urate level as seen with a change in diet, decrease in alcohol consumption), although hyperuricaemia will be demonstrated at some stage. Diagnosis is therefore usually made on clinical grounds. Definitive diagnosis: Examination of synovial fluid under polarizing light microscope for monosodium urate crystals (needle shaped and strongly negatively birefringent). - 4 - Therapeutic agents used in gout and hyperuricaemia: Three groups of drugs are available: - - - 1. Allopurinol: - Allopurinol (structural analogue of hypoxanthine), and its major metabolite, oxypurinol, inhibit the enzyme xanthine oxidase, producing a decrease in the plasma and urinary concentrations of urate (hypoxanthine does not accumulate if the salvage pathway is intact). - Initial treatment with allopurinol should be covered with an anti-inflammatory agent, because an acute attack of gout can be precipitated when the initial dose is given (sudden decrease in urate can cause mobilisation from body pools). 2. Uricosuric agents: - These drugs (eg Probenecid) increase the urinary excretion of urate by inhibiting tubular reabsorption. 3. Anti-inflammatory agents: - These agents (eg colchicine and indomethacin) are used symptomatically to relieve the pain of acute gouty arthritis. - They have no effect on plasma urate levels. INBORN ERRORS OF PURINE METABOLISM: A. Hypoxanthine-guanine phosphoribosyl transferase (HGPRT) deficiency (LeschNyhan syndrome): - The Lesch-Nyhan syndrome is an X-linked recessive disorder, due to severe deficiency of HGPRT. It is characterised by hyperuricaemia, mental deficiency, spasticity, choreoathetosis and self-mutilation. Hyperuricaemia is due to decreased activity of the salvage pathway causing decreased purine reutilization and increased uric acid synthesis. Relatively low levels of nucleotides result in decreased inhibition of de novo synthesis, resulting in further overload of the non-functioning salvage pathway and increased uric acid production. B. Glucose 6-phosphatase deficiency (Glycogen storage disease type I/ Von Gierke’s disease): (see figure 4) - Deficiency of glucose 6-phosphatase (final enzyme in glycogenolysis pathway) results in accumulation of glycogen, and hypoglycemia. Increased metabolism of glucose 6-phosphate through glycolysis results in lactic acidosis. Increased metabolism of glucose 6-phosphate through pentose phosphate pathway increases formation of ribose 5-phosphate and NADPH. Ribose 5-phosphate is a substrate for increased de novo purine nucleotide synthesis, which is subsequently degraded to uric acid resulting in hyperuricaemia. NADPH is a coenzyme in triglyceride synthesis, and overproduction results in hypertriglyceridaemia. Hyperuricaemia is aggravated by increased lactic acid which inhibits renal excretion of uric acid. 5 Figure 4: Carbohydrate metabolism Glycogen Glycogenolysis Glucose 6-phosphatase Pentose phosphate pathway Glucose 6-phosphate Glucose Glycolysis Ribose 5phosphate NADPH Purine synthesis + degradation TGS synthesis Pyruvate Lactate Uric acid C. Phosphoribosylpyrophosphate (PRPP) synthetase variant (with increased activity) - An X-linked disorder resulting in purine overproduction and gout, due to excessive activity of PRPP synthetase (first enzyme in de novo synthesis pathway) (see figure 5). This increased activity seems to be due to resistance to negative feedback inhibition by purine nucleotides. Gouty arthritis and urate lithiasis associated with hyperuricaemia occurs in childhood or early adult life. Figure 5: De novo synthesis of purine nucleotides: 6 LABORATORY INVESTIGATION: Useful estimations in the evaluation of hyperuricaemia and gout are plasma urate, plasma creatinine (renal function) and urinary urate. A. Plasma urate: Plasma urate is influenced by a wide variety of factors which should be taken into account when interpreting results: - Race: Markedly raised in Maoris. - Sex: 0.05-0.10 mmol/l higher in males (? Increased renal clearance in females, ? increased body mass in males). - Age: Higher in older age groups. - Body mass: Elevated in obesity (? Reflection of dietary habits). - Diet: Increased values in: - High meat (purine) intake - Alcohol ingestion : - Associated lactic acidosis decreases renal excretion. - Alcohol increases ATP turnover. - Purines in beer yeast. - Fasting (ketones inhibit renal urate excretion and there is increased purine degradation). - Exercise: Increase (lactate effect). - Pregnancy: - Levels initially fall by up to 25% in the first trimester, but then rise to values 20% higher than in the non-pregnant state. - Commonly used reference values: - Adult females: 0.21-0.36 mmol/l - Adult males: 0.31-0.47 mmol/l. B. Urinary urate: - Hyperuricaemia may be due to overproduction or decreased renal excretion or both. The rate of renal urate excretion provides a rough index of the production rate, provided renal function is normal. Normal excretion rate: < 6.0 mmol/day (normal diet) / < 3.5 mmol/day (low purine, alcohol free diet for 5-7 days’ duration). Values in excess of the above are presumptive evidence of urate overproduction. (see case studies) C. Plasma creatinine: - Hyperuricaemia may cause renal failure and renal failure will result in hyperuricaemia. In renal insufficiency: - The plasma urate usually does not begin to rise until the GFR (glomerular filtration rate) falls to below 20 ml/min (equivalent to a plasma creatinine of 300-400 umol/l). - As renal failure progresses the plasma urate rises to a level of around 0.6 mmol/l and then plateaus; thus a urate in excess of 0.6 mmol/l suggests that renal failure is not the only cause of the high urate. D. Urinary urate:creatinine ratio: - The urinary urate:creatinine ratio may be used to differentiate acute renal failure precipitated by hyperuricaemia (urate nephropathy) from renal failure due to other causes. - Urate nephropathy: urinary urate:creatinine ratio (mmol/l:mmol/l) > 0.70. - Acute renal failure due to other causes: ratio < 0.70. 7 CAUSES OF HYPERURICAEMIA: A. Physiological/environmental factors See above B. Primary hyperuricaemia Overproduction: - Idiopathic - Glucose-6-phosphatase deficiency (Von Gierke’s disease) - HGPRT deficiency (Lesch-Nyhan syndrome) Reduced excretion: - Idiopathic C. Secondary hyperuricaemia Overproduction: - Increased nucleic acid turnover: - Myeloproliferative disease, eg polycythemia vera - Lymphoma, leukemia - Multiple myeloma - Cytotoxic therapy of malignancies - Psoriasis - Disordered ATP metabolism: - Alcohol (increased ATP turnover) - Tissue hypoxia - Excessive dietary purine intake Reduced excretion: - Decreased glomerular filtration: - Renal failure - Decreased secretion (competition with urate for tubular secretion): - Lactic acidosis – alcohol, exercise - Ketoacidosis – alcohol, diabetes, starvation - Drugs – low dose salicylate - Increased reabsorption: - Hypovolemia, eg diuretics. CASE STUDIES: Case 1: A man aged 50 years with an acutely swollen and painful right knee. On careful history taking and thorough physical examination no secondary causes were found. Plasma Urate Creat 0.71 mmol/l (0.31-0.47) 120 umol/l (60-120) Urine Urate (purine free diet) 10.5 mmol/day (< 3.5) Questions: a) What is your diagnosis based on clinical information and laboratory investigations? b) Is the patient an “overproducer” or “undersecretor”? c) What is the treatment of choice in this patient? d) What further investigation would you like to perform to confirm the diagnosis? 8 Case 2: Acute myeloid leukemia in a 26-year-old woman. Date 23/4 Plasma Urate Creat 0.92 90 Urine Urate (normal diet) 10.5 26/4 0.18 mmol/l 110 umol/l - mmol/day (0.21-0.36) (60-120) (<6.0) Allopurinol was begun on 23/4. Questions: a) What is your diagnosis based on clinical information and laboratory investigations? b) Is the patient an “overproducer” or “undersecretor”? Case 3: Chronic renal failure in a 39-year-old man. Plasma Urate Creat 0.78 0.90 mmol/l (0.31-0.47) mmol/l (60-120) Urine Creat Clearance Urate (normal diet) 10 1.2 ml/min (> 120) mmol/day (< 6.0) Questions: a) What is your diagnosis based on clinical information and laboratory investigations? b) Is the patient an “overproducer” or “undersecretor”? c) Is the plasma urate level appropriate for the degree of renal failure? REFERENCES: 1. Walmsley RN, White GH. A guide to diagnostic clinical chemistry. 3rd edition. Oxford: Blackwell Scientific Publications, 1994: 416-425. 2. Marshall WJ. Clinical Chemistry. 4th edition. Edinburgh: Mosby, 2000: 254-260.