Cloning The Insulin Gene
advertisement

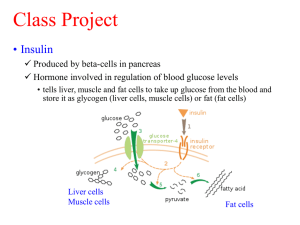
CLONING THE INSULIN GENE What Is Insulin? Most of the pancreas is a digestive gland secreting enzymes to help digest a meal. However, scattered through the pancreas are several hundred thousand clusters of special Islet cells that secrete insulin. This hormone consists of an alpha chain of 21 amino acids linked by two disulfide (S-S) bridges to a beta chain of 30 amino acids. It is secreted in response to a rise in blood glucose levels & this small protein stimulates liver & muscle cells to remove glucose from the blood and convert it into glycogen, an insoluble storage compound. In Type 1 diabetes mellitus an autoimmune attack in childhood kills the Islet cells so there is little or no insulin. The sugar continues to circulate in the blood in such large volumes that the kidneys can’t filter it out producing a large volume of urine (due to osmotic effects) with a lot of sugar in it. This disease is as old as written records. It affects about 0.5 % of the population & is rapidly increasing in incidence. The disease can be controlled by insulin, but cannot be taken by mouth (as a protein, it would be digested) so it must be injected. Prior to insulin’s discovery by Banting & Best in 1921 diabetes was invariably fatal – usually within a matter of weeks. For many years insulin was extracted from the pancreas of dogs, cows and pigs by laborious chemical methods. Unfortunately it proved too expensive to provide insulin for many in poorer countries & even today a large number of young people die of diabetes because of the lack of insulin. The molecular structure of insulin was determined in 1955 by Frederick Sanger - the first protein to be deciphered – for which he received a Nobel prize. Pig insulin was then seen to differ from human insulin by one amino acid; beef insulin by three. Although both work in humans to lower blood sugar, they are seen by the immune system as "foreign" and induce an antibody response in the patient that blunts their effect and requires higher doses. Two approaches have been tried to solve this problem. Pig insulin can be converted into human insulin by removing the one amino acid that distinguishes them and replacing it with the human version. This is, however, prohibitively expensive so now the favored approach is to genetically engineer bacteria to make the protein. Cloning The Insulin Gene In the early 1970s, biochemists at Stanford University were the first to recombine the DNA of one microbe with the inserted DNA sequence of another, in effect “editing” the DNA of the microbe (genetically modifying it). The actual editing, or insertion process, is painstaking, for it involves manipulating incredibly tiny pieces of incredibly tiny organisms. But the process can be explained in terms of editing a written text: “scissors” and "glue" are used to "cut" and "paste." Escherishia coli bacteria What are the scissors that cut the DNA open? What is the glue that joins the 2 different bits of DNA? The methods used in this Recombinant DNA (rDNA) technology are fairly simple. It involves the insertion of the gene coding for human insulin into a plasmid, which in turn carries the gene into a replicating E. coli bacterium that produces human insulin. Plasmids are small circles of double stranded DNA found in bacteria, separate from the bacterial chromosome and smaller than it. They are able to pass readily from one cell to another, even when the cells are from very different species. Consequently, plasmids can be used as vectors, to carry foreign DNA into a bacterium & replicate/clone it. How is this recombinant insulin gene cloned? 1. RNA that codes for insulin is extracted from pancreatic cells. 2. A complimentary strand of DNA (cDNA) is built onto the RNA using the enzyme reverse transcriptase. 3. Enzymes split the cDNA & RNA strands. 4. The addition of nucleotides & DNA polymerase to the single cDNA strand build a 2 nd strand on it. This DNA has special linking sequences built onto each end so it will fit into the plasmid the right way round. 5. The DNA is inserted into a plasmid which also contains a gene for resistance to an antibiotic. To do this a restriction enzyme cuts open the plasmid (the “scissors”), the cDNA is inserted & DNA ligase “glues” the 2 different DNA’s together. This recombinant plasmid now contains a human gene. 6. The plasmid is mixed in with E. coli & is transfected into the bacteria. 7. Each plasmid replicates, making many copies inside its bacterium (i.e. cloning the gene). 8. The bacteria are put into the antibiotic – only those with the insulin DNA inserted into their plasmid will survive. This is how the transfected bacteria are separated from bacteria without the recombinant DNA in their plasmids. 9. The bacterial culture goes into a fermentation tank where the bacteria (& their plasmids) reproduce. With this recombinant plasmid DNA E. coli can process the instructions to assemble the amino acids for insulin production & they make lots. The trade name for insulin produced from GE E. coli is Humulin. Yeast is also used (trade name = Novolin). This highly simplified description of rDNA technology does not fully convey the enormous complexity and awesome economy and efficiency of genetic processes. But we can begin to understand how, by using rDNA, it is possible to produce substances of medical and economic value. NZ has 15,000 diabetics that depend on bacteria genetically modified to carry the human gene that produces insulin. This GE insulin has been in use since 1981 with no adverse reactions despite very close scrutiny. Other chemicals manufactured in this way include: Factor VIII for haemophilia A Factor IX for haemophilia B Human growth hormone Erythropoietin (EPO) to boost red blood cells for anaemia Interferons & interleukins for cancer Vaccine antigens for AIDS, malaria, hepatitis.