here.
advertisement

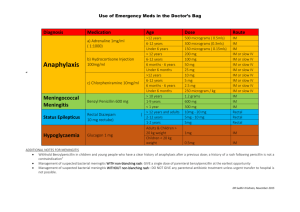
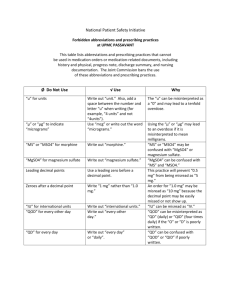
Pfizer Limited Walton Oaks, Dorking Road, Walton on the Hill, Tadworth, Surrey KT20 7NS, UK Telephone: +44 (0)1304 616161 Worldwide Biopharmaceutical Businesses Dear Healthcare Professional, You are invited to attend a meeting entitled “A local COPD update including an introduction to the new, once daily LAMA/LABA combination Ultibro® Breezhaler®▼ (glycopyrronium bromide / indacaterol maleate)” organised and funded by Pfizer on Thursday 15th October 2015 in a private meeting room at The Cromwell Hotel, High St, Stevenage, SG1 3AZ The key speakers at the meeting will be Dr Mehul Patel, Respiratory Consultant at Lister Hospital, Stevenage and Mr Darush Attar-Zadeh, Pharmacist and National Public Health Trainer. Details of how to register for the meeting are included on the following page. I hope that you will be able to attend what should be an informative and interesting meeting. Yours sincerely, Ravi Nahar Respiratory Network Sales Specialist This meeting is organised by Pfizer on behalf of the Novartis - Pfizer Alliance Page 1 of 4 UK/MTGGIPR/15-0248 August 2015 REG08-WI-GBR09-RF11b 1.0 Registered in England: No 526209 Registered Office: Ramsgate Road Sandwich, Kent CT13 9NJ, UK ULTIBRO® BREEZHALER® ▼ PRESCRIBING INFORMATION 85 micrograms/43 micrograms inhalation powder, hard capsules (indacaterol/glycopyrronium) Refer to Ultibro® Breezhaler® Summary of Product Characteristics (SmPC) before prescribing. Indications: Ultibro Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Presentation: Hard capsules for inhalation containing 110 micrograms indacaterol and 50 micrograms glycopyrronium, with the delivered dose equivalent to 85 micrograms of indacaterol and 43 micrograms of glycopyrronium. Dose and administration: The recommended dose is the inhalation of the content of one capsule once a day using the Ultibro Breezhaler inhaler. Ultibro Breezhaler should be administered at the same time of day each day. No dose adjustment is required for elderly patients or patients with mild to moderate renal impairment or patients with mild to moderate hepatic impairment. There is no relevant use of Ultibro Breezhaler in patients under 18 years. Ultibro Breezhaler capsules are for inhalation use only and must not be swallowed. Contraindications: Hypersensitivity to the active substance, lactose monohydrate or magnesium stearate. Precautions: Ultibro Breezhaler should not be used for the treatment of asthma. Ultibro Breezhaler is not indicated for the treatment of acute episodes of bronchospasm, i.e. as a rescue therapy. In clinical studies with Ultibro Breezhaler, paradoxical bronchospasm was not observed. However, paradoxical bronchospasm has been observed with other inhalation therapy and can be life-threatening. If this occurs, Ultibro Breezhaler should be discontinued immediately. Immediate hypersensitivity reactions have been reported after administration of Ultibro Breezhaler components. If signs suggesting allergic reactions occur, in particular, angioedema, urticaria or skin rash, Ultibro Breezhaler should be discontinued immediately and alternative therapy instituted. Ultibro Breezhaler should be used with caution in patients with narrow-angle glaucoma or urinary retention. Patients should be informed about the signs and symptoms of acute narrow-angle glaucoma and should be informed to stop using Ultibro Breezhaler and contact their doctor immediately should this event occur. In patients with severe renal impairment, including those with end-stage renal disease requiring dialysis, Ultibro Breezhaler should be used only if the expected benefit outweighs the potential risk and these patients should be monitored closely for potential adverse reactions. Ultibro Breezhaler should be used with caution in patients with a history of cardiovascular disorders such as coronary artery disease, acute myocardial infarction, cardiac arrhythmias and hypertension. Beta2-adrenergic agonists may produce a clinically significant cardiovascular effect in some patients; if such effects occur, treatment may need to be discontinued. Patients with unstable ischaemic heart disease, left ventricular failure, history of myocardial infarction, arrhythmia (excluding chronic stable atrial fibrillation), a history of long QT syndrome or whose QTc was prolonged were excluded from the clinical trials, and as there is no experience in these patient groups, Ultibro Breezhaler should be used with caution. Upon initiation of treatment plasma glucose should be monitored more closely in diabetic patients. Use with caution in patients with convulsive disorders or thyrotoxicosis. Patients with rare hereditary problems of galactose intolerance, the Lapp lactose deficiency or glucose-galactose malabsorption should not take this medicine. Drug interactions: Concomitant administration of orally inhaled indacaterol and glycopyrronium, under steady-state conditions of both components, did not affect the pharmacokinetics of either component. No specific interaction studies were conducted for Ultibro Breezhaler. Information on the potential for interactions is based on the potential for each individual component. The concomitant use of Ultibro Breezhaler with beta-adrenergic blockers, anticholinergics or sympathomimetic agents is not recommended. Caution is required with the concomitant use of hypokalaemic treatment. Pregnancy and lactation: There are no data from the use of Ultibro Breezhaler in pregnant women. Indacaterol may inhibit labour due to a relaxant effect on uterine smooth muscle and therefore Ultibro Breezhaler should only be used during pregnancy if the expected benefit to the patient justifies the potential risk to the foetus. The use of Ultibro Breezhaler by breast-feeding women should only be considered if the expected benefit to the woman is greater than any possible risk to the infant. Effects on ability to drive and use machines: This medicinal product has no or negligible influence on the ability to drive and use machines. However, the occurrence of dizziness may influence the ability to drive and use machines. Undesirable effects: Very common (≥1/10): upper respiratory tract infection. Common (≥1/100 to <1/10): nasopharyngitis, urinary tract infection, sinusitis, rhinitis, dizziness, headache, cough (usually of mild intensity), oropharyngeal pain (including throat irritation), dyspepsia, dental caries, gastroenteritis, musculoskeletal pain, pyrexia and chest pain. Uncommon (≥1/1000 to <1/100: Hypersensitivity, angioedema, diabetes mellitus and hyperglycaemia, insomnia, paraesthesia, glaucoma, ischaemic heart disease, atrial fibrillation, tachycardia, palpitations, paradoxical bronchospasm, epistaxis, dry mouth, pruritus/rash, muscle spasm, myalgia, pain in extremity, bladder obstruction and urinary retention, peripheral oedema, fatigue. Ultibro Breezhaler showed similar adverse reactions to the individual components; indacaterol and glycopyrronium, and as such, the type and severity of adverse reactions associated with each of these components may be expected in the combination. Quantities and basic NHS price (excl. VAT): Ultibro Breezhaler with 30 day supply of 85 micrograms/43 micrograms capsules: £32.50. Ultibro Breezhaler with 12 day supply of 85 micrograms/43 micrograms capsules: £13.00. Marketing authorisation number: EU/1/13/862/002, 003 Legal category: POM. Date of last revision of prescribing information: September 2015. UB05 Full prescribing information is available from Novartis Pharmaceuticals UK Ltd, Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR. Telephone: 01276 698370. Email: medinfo.uk@novartis.com Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis 01276 698370 INVITATION TO “A local COPD update including an introduction to the new, once daily LAMA/LABA combination Ultibro® Breezhaler® ▼ (glycopyrronium bromide/indacaterol maleate)” On Thursday 15th October 2015 at 18.30 To be held at The Cromwell Hotel, High St, Stevenage, SG1 3AZ Agenda 18.30 Arrival, Registration and Hot Buffet 19.00 “A local COPD update including an introduction to the new, once daily LAMA/LABA combination Ultibro® Breezhaler® ▼ (glycopyrronium bromide/indacaterol maleate)” Dr Mehul Patel, Respiratory Consultant, Lister Hospital, Stevenage. 19.45 Question and Answer Session 20.00 Coffee 20.10 “Making Every Contact Count” Mr Darush Attar-Zadeh, Pharmacist and National Trainer in Smoking Cessation. 21.00 Questions and Answers Session 21.15 Summary and Close This meeting is organised & funded by Pfizer Ltd on behalf of the Novartis - Pfizer Alliance. Please note that, under the law and the ABPI Code of Practice, Pfizer may only provide hospitality and/or promote its medicines to members of the healthcare professions and appropriate administrative staff. Therefore, no unqualified person (e.g. non-medical spouses, partners) may be invited to or attend Pfizer meetings. …………………………………………………………………………… REPLY* Please reply to confirm your attendance at this meeting; By email: ravinder.nahar@pfizer.com Alternatively, you can respond by telephone to: Ravi Nahar - 07880256994 (Please include the meeting date and any special dietary requirements in your response). To contact Pfizer for any other purpose, including adverse event reports or medical information requests, please call 01304 616161 *By providing your personal information, you agree that the information will be held on a database (located within or outside the EU) controlled by Pfizer Limited and used for the purpose of meeting registration and contacting you about similar events in the future. Only Pfizer, organisations working with Pfizer in the administration of your information, and Pfizer and Novartis group companies will have access to your information. Pfizer will always employ appropriate technical security measures to protect your personal information and to ensure that it is not accessed by unauthorised persons. You will at any time have the right to access your information, you can request that your information on the Pfizer database is amended, or you can unsubscribe from any programme at any time by emailing us at: DataProtectionUK@Pfizer.com. UK/MTGGIPR/15-0248 August 2015 REG08-WI-GBR09-RF11b 1.0 Page 3 of 4 ULTIBRO® BREEZHALER® ▼ PRESCRIBING INFORMATION 85 micrograms/43 micrograms inhalation powder, hard capsules (indacaterol/glycopyrronium) Refer to Ultibro® Breezhaler® Summary of Product Characteristics (SmPC) before prescribing. Indications: Ultibro Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD). Presentation: Hard capsules for inhalation containing 110 micrograms indacaterol and 50 micrograms glycopyrronium, with the delivered dose equivalent to 85 micrograms of indacaterol and 43 micrograms of glycopyrronium. Dose and administration: The recommended dose is the inhalation of the content of one capsule once a day using the Ultibro Breezhaler inhaler. Ultibro Breezhaler should be administered at the same time of day each day. No dose adjustment is required for elderly patients or patients with mild to moderate renal impairment or patients with mild to moderate hepatic impairment. There is no relevant use of Ultibro Breezhaler in patients under 18 years. Ultibro Breezhaler capsules are for inhalation use only and must not be swallowed. Contraindications: Hypersensitivity to the active substance, lactose monohydrate or magnesium stearate. Precautions: Ultibro Breezhaler should not be used for the treatment of asthma. Ultibro Breezhaler is not indicated for the treatment of acute episodes of bronchospasm, i.e. as a rescue therapy. In clinical studies with Ultibro Breezhaler, paradoxical bronchospasm was not observed. However, paradoxical bronchospasm has been observed with other inhalation therapy and can be lifethreatening. If this occurs, Ultibro Breezhaler should be discontinued immediately. Immediate hypersensitivity reactions have been reported after administration of Ultibro Breezhaler components. If signs suggesting allergic reactions occur, in particular, angioedema, urticaria or skin rash, Ultibro Breezhaler should be discontinued immediately and alternative therapy instituted. Ultibro Breezhaler should be used with caution in patients with narrow-angle glaucoma or urinary retention. Patients should be informed about the signs and symptoms of acute narrow-angle glaucoma and should be informed to stop using Ultibro Breezhaler and contact their doctor immediately should this event occur. In patients with severe renal impairment, including those with end-stage renal disease requiring dialysis, Ultibro Breezhaler should be used only if the expected benefit outweighs the potential risk and these patients should be monitored closely for potential adverse reactions. Ultibro Breezhaler should be used with caution in patients with a history of cardiovascular disorders such as coronary artery disease, acute myocardial infarction, cardiac arrhythmias and hypertension. Beta2-adrenergic agonists may produce a clinically significant cardiovascular effect in some patients; if such effects occur, treatment may need to be discontinued. Patients with unstable ischaemic heart disease, left ventricular failure, history of myocardial infarction, arrhythmia (excluding chronic stable atrial fibrillation), a history of long QT syndrome or whose QTc was prolonged were excluded from the clinical trials, and as there is no experience in these patient groups, Ultibro Breezhaler should be used with caution. Upon initiation of treatment plasma glucose should be monitored more closely in diabetic patients. Use with caution in patients with convulsive disorders or thyrotoxicosis. Patients with rare hereditary problems of galactose intolerance, the Lapp lactose deficiency or glucose-galactose malabsorption should not take this medicine. Drug interactions: Concomitant administration of orally inhaled indacaterol and glycopyrronium, under steady-state conditions of both components, did not affect the pharmacokinetics of either component. No specific interaction studies were conducted for Ultibro Breezhaler. Information on the potential for interactions is based on the potential for each individual component. The concomitant use of Ultibro Breezhaler with beta-adrenergic blockers, anticholinergics or sympathomimetic agents is not recommended. Caution is required with the concomitant use of hypokalaemic treatment. Pregnancy and lactation: There are no data from the use of Ultibro Breezhaler in pregnant women. Indacaterol may inhibit labour due to a relaxant effect on uterine smooth muscle and therefore Ultibro Breezhaler should only be used during pregnancy if the expected benefit to the patient justifies the potential risk to the foetus. The use of Ultibro Breezhaler by breast-feeding women should only be considered if the expected benefit to the woman is greater than any possible risk to the infant. Effects on ability to drive and use machines: This medicinal product has no or negligible influence on the ability to drive and use machines. However, the occurrence of dizziness may influence the ability to drive and use machines. Undesirable effects: Very common (≥1/10): upper respiratory tract infection. Common (≥1/100 to <1/10): nasopharyngitis, urinary tract infection, sinusitis, rhinitis, dizziness, headache, cough (usually of mild intensity), oropharyngeal pain (including throat irritation), dyspepsia, dental caries, gastroenteritis, musculoskeletal pain, pyrexia and chest pain. Uncommon (≥1/1000 to <1/100: Hypersensitivity, angioedema, diabetes mellitus and hyperglycaemia, insomnia, paraesthesia, glaucoma, ischaemic heart disease, atrial fibrillation, tachycardia, palpitations, paradoxical bronchospasm, epistaxis, dry mouth, pruritus/rash, muscle spasm, myalgia, pain in extremity, bladder obstruction and urinary retention, peripheral oedema, fatigue. Ultibro Breezhaler showed similar adverse reactions to the individual components; indacaterol and glycopyrronium, and as such, the type and severity of adverse reactions associated with each of these components may be expected in the combination. Quantities and basic NHS price (excl. VAT): Ultibro Breezhaler with 30 day supply of 85 micrograms/43 micrograms capsules: £32.50. Ultibro Breezhaler with 12 day supply of 85 micrograms/43 micrograms capsules: £13.00. Marketing authorisation number: EU/1/13/862/002, 003 Legal category: POM. Date of last revision of prescribing information: September 2015. UB05 Full prescribing information is available from Novartis Pharmaceuticals UK Ltd, Frimley Business Park, Frimley, Camberley, Surrey GU16 7SR. Telephone: 01276 698370. E-mail: medinfo.uk@novartis.com Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis 01276 698370