1- Propal alcohol (C3H7OH) is burned with 50 percent excess air
advertisement

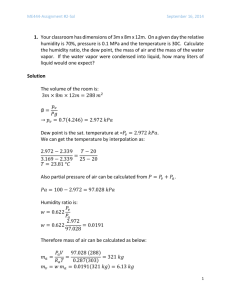
1- Propal alcohol (C3H7OH) is burned with 50 percent excess air. Write the balanced reaction equation for complete combustion and determine the air-to-fuel ratio. 2- A gaseous fuel with a volumetric analysis of 60 percent CH4, 30 percent H2, and 10 percent N2 is burned to completion with 130 percent theoretical air. Determine (a) the air– fuel ratio and (b) the fraction of water vapor that would condense if the product gases were cooled to 20°C at 1 atm. 3- Methane (CH4) is burned with dry air. The volumetric analysis of the products on a dry basis is 5.20 percent CO2, 0.33 percent CO, 11.24 percent O2, and 83.23 percent N2. Determine (a) the air–fuel ratio and (b) the percentage of theoretical air used. 4-A gaseous fuel with 80 percent CH4, 15 percent N2, and 5 percent O2 (on a mole basis) is burned with dry air that enters the combustion chamber at 25°C and 100 kPa. The volumetric analysis of the products on a dry basis is 3.36 percent CO2, 0.09 percent CO, 14.91 percent O2, and 81.64 percent N2. Determine (a) the air–fuel ratio, (b) the percent theoretical air used, and (c) the volume flow rate of air used to burn fuel at a rate of 1.4 kg/min. 5- The furnace of a particular power plant can be considered to consist of two chambers: an adiabatic combustion chamber where the fuel is burned completely and adiabatically and a counterflow heat exchanger where heat is transferred to a reversible heat engine. The mass flow rate of the working fluid of the heat engine is such that the working fluid is heated from T0 (the temperature of the environment) to Taf (the adiabatic flame temperature) while the combustion products are cooled from Taf to T0. Treating the combustion products as ideal gases with constant specific heats and assuming no change in their composition in the heat exchanger, show that the work output of this reversible heat engine is: 6دماي آدياباتيك شعله حاصل از احتراق متان مايع با %400 هواي تئوري در دماي 25درجه سلسيوس را تعيين كنيد. -7پروپان مايع ( )C3H8در دماي 25درجه سلسيوس و با دبي 0/05كيلوگرم بر دقيقه وارد محفظه احتراق ميشود و با %50 هواي اضافي كه در دماي 7درجه سلسيوس ميباشد ،مخلوط شده و سوزانده ميشود .تجزيه محصوالت نشان ميدهد كه تمام هيدروژن موجود در سوخت ميسوزد؛ اما تنها %90از كربن كامل ميسوزد و به دياكسيد كربن تبديل ميشود و %10 باقيمانده به مونوكسيد كربن تبديل ميشود .الف) نشان دهيد كه اگر دماي خروجي گازهاي احتراق 1500كلوين باشد، واكنش اشاره شده خود به خودي است .ب) دماي آدياباتيك شعله را بدست آوريد و آن را با دماي محصوالت احتراق واكنش باال مقايسه كنيد؟ ج) اگر به جاي هوا از اكسيژن خالص استفاده شود دماي احتراق چه تغييري ميكند؟