Energy Content of Fuels - Fairmont State College

Energy Content of Fuels
In this experiment, you will find and compare the enthalpy of combustion of different fuels: paraffin and ethanol. Paraffin is a member of a group of compounds called alkanes that are composed entirely of carbon and hydrogen atoms, such as C
22
H
46
. Many alkanes, such as gasoline and diesel oil, are important fuels. Ethanol, C2H5OH, is used as a gasoline additive (gasohol) and as a gasoline substitute. In this experiment, you will compare the energy content of paraffin and ethanol by measuring their enthalpy of combustion in kJ/g of fuel.
In order to find the enthalpy of combustion, you will first use the energy from burning ethanol or paraffin to heat a known quantity of water. By monitoring the temperature of the water, you can find the amount of heat transferred to it, using the general formula q = Cp
• •
Where q is the heat transferred to an object, Cp is the specific heat capacity of the object, m is the mass of the object, and
can. Finally, the amount of fuel burned will be taken into account by calculating the heat per gram of fuel consumed in the combustion.
PROCEDURE 1: Paraffin Wax
Prepare a candle for the experiment by holding a lighted match near its base, so that some melted f l ont 3” 5” i for a moment to fasten it to the card. Light the candle wick and keep it lit for 30 seconds, then extinguish it. This step ensures that the wick is primed, and will not be burning itself. Find and record the combined mass of the candle and index card (or the alcohol burner and contents in Procedure 2 below).
Determine and record the mass of an empty can. Add 100 mL of chilled tap water to the can (use
200 mL of chilled water in Procedure 2). Determine and record the mass of the can and water.
Prepare the computer for time-based data collection (set collection time to 1000 seconds) by calibrating the temperature probe with ice/water and room-temperature baths.
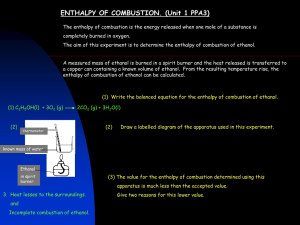
Use a ring and stirring rod to suspend the can 2-3 cm above the wick. Use the slit stopper to suspend the temperature probe in the water. The probe should not touch the bottom of the can. Wait at least 60 seconds for the thermocouple to adjust to the temperature of the water.
Begin measuring temperature. After the computer has taken readings for about 30 seconds, light the candle (or alcohol burner in Procedure 2). Adjust the height of the can so that the tip of the flame just touches the bottom of the can. Heat the water until its temperature reaches 40°C and then extinguish the flame. Stir the water until the temperature remains constant for ten seconds, and then stop data collection.
Determine and record the final mass of the cooled candle and index card, including all drippings
(or the cooled alcohol burner and contents in Procedure 2). Using the cursor, find the starting temperature and final temperature of the water in the can. Also record the time difference between the starting the heating and reaching the maximum temperature.
PROCEDURE 2: Ethanol
Repeat the procedure using ethanol in an alcohol burner. Be sure to use 200 mL of chilled water.
Before assembling the apparatus, light the alcohol burner and keep lit for 30 seconds, then extinguish.
PROCESSING THE DATA
1. For each fuel, calculate the heat (q) absorbed by the water in the can. For water, Cp = 4.18 J/g °C.
Change your value of q to kJ.
2. Using the mass of paraffin or ethanol burned; calculate the enthalpy of combustion, per gram of fuel burned, for paraffin and ethanol (in kJ/g fuel). Based on your results, which fuel produces more energy per gram burned?
3. If 12 g of paraffin were burned to warm 5.0 L of water with an initial temperature of 4.0
˚
, what would be the final temperature of the water? (
final
–
T initial
)
4. For each fuel, calculate the heat produced per mole of fuel that was burned.
5. By investigating the heating curves for the two fuels, determine which fuel produced the greatest amount of heat per unit time (not per unit mass).
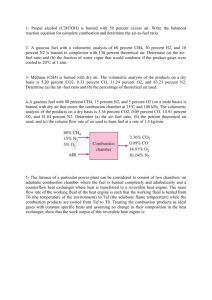
6. Write balanced chemical equations for the burning of paraffin and ethanol.
7. How many grams of oxygen from the air were used in burning each of your fuels?
pt d om mi t t Comput
Vernier Software, Portland, OR 97225 s or he h, Da mqui t Dona d Vol ,
Exam Practice Questions
Outcome 19
This is an algebra outcome, so you must show equations, and manipulate them before plugging in numbers. Get in this habit. You do not have to solve your equations simultaneously.
1. 1.50 kg of water at 25 o
C is heated by burning 8.31 grams of methane gas. What is the final temperature of the water after the methane is combusted? Assume no heat losses to the surroundings. The heat capacity of water is 4.184 J/g C.
CH
4
(g) + 2 O
2
(g)
CO
2
(g) + 2 H
2
O (g) H rxn
= -890.5 kJ/mole rxn
2. 10.5 kg of water at 15 o
C is heated by burning 10.31 grams of C
6
H
6
(l). What is the final temperature of the water after the C
6
H
6
(l) is combusted? Assume no heat losses to the surroundings. The heat capacity of water is 4.184 J/g C.
C
6
H
6
(l) + 15 O
2
(g) 12 CO
2
(g) + 6 H
2
O (g) H rxn
= -6271. kJ/mole
3. 1000. grams of water at 10.00
o
C is heated by burning 1.63 grams of H
2
(g). What is the final temperature of the water after the hydrogen is combusted? Assume no heat losses to the surroundings. The heat capacity of water is 4.184 J/g C.
2 H
2
(g) + O
2
(g)
2 H
2
O (g) H rxn
= -483.6 kJ
4. The combustion of 2.57 g sample nicotine C
8
H
10
N
4
O
2 is used to heat a calorimeter at 19.4
with a mass of 1.56 kg and heat capacity (or specific heat) of 3.65 J/g C. What is the final temperature of the calorimeter, assume no heat loss to the surroundings o
C
2 C
8
H
10
N
4
O
2
+ 27O
2
16 CO
2
+ 10 H
2
O + 8 NO
2
ΔH rxn
= -4952.2kJ/mole rxn