Effect of Temperature on Equilibrium
advertisement

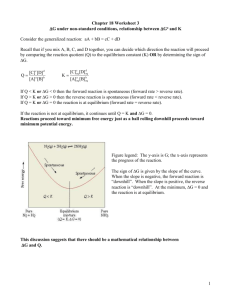
Derivation of the equilibrium constant expression for a multi-step mechanism This derivation is essentially identical to what we did in class assuming a one-step mechanism. Consider the following proposed mechanism for the formation of water vapor from hydrogen gas and oxygen gas: Step 1: H2 + O2 = 2OH Step 2: H2 + OH = H2O + H Step 3: H + OH = H2O Net: 2H2 + O2 = 2H2O Let k1 and k-1 be the rate constants for the forward and reverse reactions in step 1 Let k2 and k-2 be the rate constants for the forward and reverse reactions in step 2 Let k3 and k-3 be the rate constants for the forward and reverse reactions in step 3 When the net reaction is at equilibrium, all 3 steps in the mechanism must also be at equilibrium. So for each step: rate of forward reaction = rate of reverse reaction k1[H2][O2] = k-1[OH]2 k2[H2][OH] = k-2[H2O][H] k3[H][OH] = k-3[H2O] Remember that I can write the rate equations only for the elementary steps, NOT for the net reaction. Keq1 k1 [OH ] 2 k 1 [ H 2 ][O2 ] Keq2 k 2 [ H 2 O][ H ] k 2 [ H 2 ][OH ] Keq2 k3 [ H 2 O] k 3 [ H ][OH ] k k k [OH ]2 [ H 2 O][ H ] [ H 2 O] [ H 2 O] 2 Keqnet 1 2 3 2 k 1 k 2 k 3 [ H 2 ][O2 ] [ H 2 ][OH ] [ H ][OH ] [ H 2 ] [O2 ] Notice that this is very similar to our previous result with a one-step mechanism but now the equilibrium constant is a combination of six rate constants rather than two. The bottom line is: regardless of the mechanism, for the reaction: aA + bB = cC + dD Keq = d [C ]ceq [ D ]eq a [ A]eq [ B ]beq is a constant because it is just some combination of rate constants. Since rate constants depend on temperature and on the presence of a catalyst, you might expect the equilibrium constants would also. Equilibrium constants do change with temperature but do not change in the presence of a catalyst. The following discussion explains why. Effect of Temperature and Catalysts on Equilibrium According to the Arrhenius equation: k = Ae-Ea/RT ln k = ln A – Ea/RT The effect of temperature on Keq At two different temperatures: ln (k2/k1) = -Ea/R(1/T2 – 1/T1) If the temperature is raised then T2 > T1 and the right side of the equation is positive. Thus k2 > k1. (Increasing the temperature increases the rate constant.) If the temperature is lowered then T2 < T1 and the right side of the equation is negative. Thus k2 < k1. (Decreasing the temperature decreases the rate constant.) The equation shows that the effect of temperature on k is proportional to the activation energy. Thus, as activation energy increases, the effect of changing temperature increases. Remember that one direction of a reaction is always exothermic and the other direction is endothermic. The endothermic direction has the larger activation energy. When temperature increases, both rates (forward and reverse) increase but the rate of the endothermic reaction increases more! Equilbrium shifts in the endothermic direction. (LeChatelier says that this consumes the added heat!) When temperature decreases, both rates (forward and reverse) decrease but the rate of the endothermic reaction decreases more! Equilbrium shifts in the exothermic direction. (LeChatelier says that this partially replenishes the removed heat!) RAISING THE TEMPERATURE INCREASES Keq FOR AN ENDOTHERMIC REACTION AND LOWERS Keq FOR AN EXOTHERMIC REACTION (LOWERING THE TEMPERATURE HAS THE OPPOSITE EFFECT.) The effect of a catalyst on Keq A catalyst lowers the activation energy. If Ea1 is the activation energy without a catalyst and Ea2 is the activation energy with a catalyst: ln (k2/k1) = -1/RT(Ea2-Ea1) Ea2 < Ea1 so the right side of the equation is positive. Thus k2 >k1 which shows that the rate increases upon addition of a catalyst. Notice that the effect of a catalyst depends only on the absolute difference between Ea2 and Ea1. This difference is the same for both the forward and reverse directions of a chemical reaction. Thus, a catalyst speeds up both directions by the same amount and does not shift the equilibrium. ADDING A CATALYST HAS NO EFFECT ON Keq