Buffering Capacity of Water
advertisement

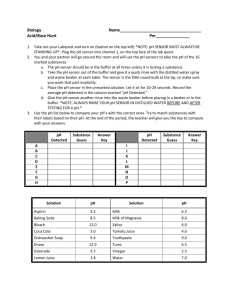
pH change of Water -Tom Green, wtgreen68@mac.com INTRODUCTION The increasing acidity of the world's oceans -- and that acidity's growing threat to marine species -- are definitive proof that the atmospheric carbon dioxide that is causing climate change is also negatively affecting the marine environment, says Antarctic marine biologist Jim McClintock, Ph.D., professor in the University of Alabama at Birmingham (UAB) Department of Biology. "The oceans are a sink for the carbon dioxide that is released into the atmosphere," says McClintock, who has spent more than two decades researching the marine species off the coast of Antarctica. Carbon dioxide is absorbed by oceans, and hydrogen ions are released to make seawater more acidic. Carbon dioxide dissolves slightly in water to form a weak acid called carbonic acid, H2CO3, according to the following reaction: CO2 + H2O --> H2CO3 After that, carbonic acid reacts slightly and reversibly in water to form a hydronium cation, H3O+, and the bicarbonate ion, HCO3-, according to the following reaction: H2CO3 + H2O --> HCO3- + H3O+ "Existing data points to consistently increasing oceanic acidity, and that is a direct result of increasing carbon dioxide levels in the atmosphere; it is incontrovertible," McClintock says. "The ramifications for many of the organisms that call the water home are profound." McClintock says data collected since the pre-industrial age indicates the mean surface pH of the oceans has declined from 8.2 to 8.1 units with another 0.4 unit decline possible by century's end. A single whole pH unit drop would make ocean waters 10 times more acidic, which could rob many marine organisms of their ability to produce protective shells -- and tip the balance of marine food chains. (ScienceDaily (Feb. 5, 2010)) Acidification of the seas linked to climate change could threaten fisheries production and is already causing the fastest shift in ocean chemistry in 65 million years, a U.N. study sowed. (Reuters, 2011) To accurately measure pH an electrode is used. The Vernier pH electrode is relatively inexpensive, accurate and durable if used and cared for properly. PURPOSE: A pH sensor will be calibrated and used to measure the pH of solutions. We will determine the effect on pH of adding carbon dioxide to distilled water and to surface water. MATERIALS: Vernier pH electrode TI-84 Plus Buffer solutions - pH 4, 7 and 10 Tissues Burette Clamp Drinking straw 50 or 100 ml Beakers (small cups) CBL 2 AC Adapter for CBL 2 Distilled water Ring stand 250-ml Beaker CARE OF THE pH SENSOR 1. Do not leave the pH sensor in distilled water for more than a few minutes. It is better to leave it sitting in tap water than in distilled water. It may be left in a pH-4 or pH-7 buffer solution for up to 24 hours. 2. For long-term storage always store the pH sensor in its storage solution. Make sure that when the sensor is put away it is sitting upright. 3. Do not place the pH sensor in concentrated acids or bases. 4. Notice the glass bulb at the end of the pH sensor. It is very fragile. PROCEDURE: 1. Obtain and wear goggles. 2. Add 125 mL of distilled water to a clean 250-mL Beaker or to a Styrofoam cup and place inside beaker for stability. 3. Clamp the pH sensor on a ring stand with a burette clamp. 4. Prepare the pH system for data collection. (a) Plug the pH system into channel 1 of the CBL2. (b) Place TI Graphing Calculator in the cradle. Use the link cable to connect the CBL2 to the TI Graphing Calculator. Firmly press in the cable ends. 5. Turn on the calculator. Press screen will appear. APPS and select EasyData. EasyData’s MAIN Calibrating the pH electrode: (Calibration is optional. To use the default calibration go on to step 8. Calibrating the pH electrode just before making measurements will improve accuracy. This is especially important if you want to make credible measurements in the field.). To save buffer use the small beakers either 50 ml or at most 100 ml. If your instructor directs you to use the stored calibration, proceed directly to the next Step. If your instructor directs you to perform a new calibration for the pH Sensor, follow this procedure. First Calibration Point a. Select from the Main screen and then select CH1:PH. b. Select from Sensor Setup screen, then select , then select Two Point Live… c. Remove the sensor from the bottle by loosening the lid, then rinse the sensor with distilled water. d. Place the sensor tip into pH 4 buffer. Wait for the displayed voltage to stabilize, then select . e. Type 4 as the pH value and then select . f. g. h. i. Second Calibration Point Rinse the pH Sensor with distilled water and place it in the pH 10 buffer solution. Wait for the displayed voltage to stabilize, then select . Type 10 as the pH value and then select . Select twice to return to the Main screen. 6. The main screen will now display in the upper right hand corner - the pH of the solution in which the pH sensor is submerged. (a) To collect data on the effect of blowing into water you will RECORD ONLY the pH at the beginning, "# of breaths" and at the end, "when the pH value is constant." (b) Try to blow out the same amount of air each time. Observe the change in pH after each breath and continue blowing until the pH doesn’t change for several breaths. 7. Now place the pH electrode into the 250 ml Beaker with 125 ml of distilled water and clamp it in place onto the ring stand. After a 60 seconds of swirling, record the pH value in the Initial pH space in Data Table 1. 8. Now blow with a straw into the flask and observe the change in pH after each breath until the pH doesn't change for 5 breaths. Record the pH value in the Final pH space in Data Table 1 and enter the TOTAL number of breaths in the appropriate space. 9. Repeat the steps 2 and 3 with 125-mL of a surface water source. Record your data in Table 1. 10. Rinse the pH electrode with distilled water and wipe with a tissue. Replace the electrode storage bottle on the electrode. Make sure that the tip of the electrode is submerged in the liquid. DATA: Sample Table 1 Initial pH Final pH # of breaths to reach stable pH Distilled Water Surface Water QUESTIONS: 1. What constituent in your breath has the primary effect on the pH when you blow into the liquids? 2. How does the data for distilled water show why unpolluted rain typically has a pH between 5 and 6? 3. How much did the pH of surface water change? Will your waters be influenced by acid rain, by increased CO2? 4. Carbon dioxide is absorbed by oceans, and through a chemical process hydrogen ions are released to make seawater more acidic. What impact does the decrease in pH on the oceans have on organisms? SAMPLE DATA Initial pH Final pH Distilled Water 5.7 4.7 13 Lake Erie 7.8 7.3 38 Sample # of breaths to reach stable pH Reference: (ScienceDaily (Feb. 5, 2010)) http://www.sciencedaily.com/releases/2010/02/100204144811.htm (Reuters, 2010) - http://uk.reuters.com/article/idUKTRE6B16MD20101202