BOX 7-1 Genetic Blocks in Lymphocyte Maturation
advertisement

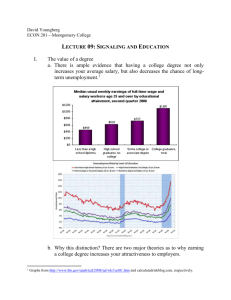
Table 11-1. Comparative Features of the Cytokines of Innate and Adaptive Immunity Features Innate immunity Adaptive immunity Examples TNF-α, IL-1, IL-12, IFN-γ * IL-2, IL-4, IL-5, IFN-γ * Major cell source Macrophages, NK cells T lymphocytes Principal physiologic Mediators of innate immunity and functions inflammation (local and systemic) Adaptive immunity: regulation of lymphocyte growth and differentiation; activation of effector cells (macrophages, eosinophils, mast cells) Stimuli LPS (endotoxin), bacterial peptidoglycans, viral RNA, T cellderived cytokines (IFN-γ) Protein antigens Amounts produced Local or systemic effects May be high; detectable in serum Both Generally low; usually undetectable in serum Usually local only Roles in disease Systemic diseases (e.g., septic shock) Local tissue injury (e.g., granulomatous inflammation) Inhibitors of synthesis Corticosteroids Cyclosporine, FK-506 *IFN-γplays important roles in innate and adaptive immunity. Table 11-2. Signal Transduction Mechanisms of Cytokine Receptors Signal transduction Cytokine receptors pathway using this pathway Signaling mechanism JAK/STAT pathway Type I and type II cytokine receptors JAK-mediated phosphorylation and activation of STAT transcription factors (see Box 11-2) TNF receptor signaling by TRAFs TNF receptor family: TNRRII, CD40 Binding of adapter proteins, activation of transcription factors (see Box 11-1) TNF receptor signaling by death domains TNF receptor family: TNF-RI, Binding of adapter proteins, caspase activation (see Fas Box 11-1) Receptor-associated tyrosine kinases M-CSF receptor, stem cell factor receptor Intrinsic tyrosine kinase activity in receptor G protein signaling Chemokine receptors GTP exchange and dissociation of Gα. GTP from Gβγ, Gα. GTP activates various cellular enzymes page 248 page 249 Table 11-3. Cytokines of Innate Immunity Cytokine Size Principal cell source Principal cell targets and biologic effects Tumor necrosis factor (TNF) 17 kD; 51-kD homotrimer Macrophages, T cells Endothelial cells: activation (inflammation, coagulation) Neutrophils: activation Hypothalamus: fever Liver: synthesis of acute-phase proteins Muscle, fat: catabolism (cachexia) Many cell types: apoptosis Interleukin-1 (IL- 17 kD mature form; Macrophages, endothelial Endothelial cells: activation (inflammation, 1) 33-kD precursors cells, some epithelial cells coagulation) Hypothalamus: fever Liver: synthesis of acute-phase proteins Chemokines 8-12 kD (see Table 11-4) Macrophages, endothelial Leukocytes: chemotaxis, activation; cells, T cells, fibroblasts, migration into tissues platelets Interleukin-12 (IL-12) Heterodimer of 35- Macrophages, dendritic kD + 40-kD cells subunits T cells: TH1 differentiation NK cells and T cells: IFN-γ synthesis, increased cytolytic activity Type I IFNs (IFN- IFN-α: 15-21 kD α, IFN-β) IFN-β: 20-25 kD IFN-α: macrophages IFN-β: fibroblasts All cells: antiviral state, increased class I MHC expression NK cells: activation Interleukin-10 (IL-10) Macrophages, T cells (mainly TH2) Macrophages, dendritic cells: inhibition of IL12 production and expression of costimulators and class II MHC molecules Homodimer of 3440 kD; 18-kD subunits Interleukin-6 (IL- 19-26 kD 6) Macrophages, endothelial Liver: synthesis of acute-phase proteins B cells, T cells cells: proliferation of antibody-producing cells Interleukin-15 (IL-15) 13 kD Macrophages, others NK cells: proliferation T cells: proliferation (memory CD8+ cells) Interleukin-18 (IL-18) 17 kD Macrophages NK cells and T cells: IFN-γ synthesis BOX 11-1 Signaling by the TNF Receptor (TNF-R) Family The TNF family of proteins includes secreted cytokines and membrane proteins that share sequence homologies and fold into homotrimeric triangular pyramidal complexes, which bind to structurally similar cell surface receptors. Members of this family include TNF, lymphotoxin (TNF-β), Fas ligand, CD40 ligand, OX-40, receptor activator of NF-κB ligand (RANK-L), TNF-related apoptosis-inducing ligand (TRAIL), and several other proteins. The cell surface receptors for the TNF family of proteins are type I membrane proteins that can also be grouped into a family, called the TNF-R family, on the basis of sequence homology in their cysteine-rich extracellular ligand-binding domains. Members of the TNF-R family include TNF-RI, TNF-RII, lymphotoxin-β receptor (LT-βR), Fas, CD40, OX-40 ligand, RANK, and others. The binding of TNF family members to their respective receptors initiates a wide variety of responses. Many of these responses are proinflammatory and depend on activation of the NF-κB and AP-1 transcription factors leading to new gene transcription. In contrast, some TNF-R family members (TNF-RI and Fas) deliver signals that cause apoptotic cell death. The specific responses to the cytokines depend on the particular receptor and cell type. Many of the molecular details of the signaling pathways engaged by TNF-R family molecules are now known. Although the members of this receptor family share structural features in their extracellular ligand-binding regions, they have widely divergent cytoplasmic domain structures. Nonetheless, there are shared features in the signaling pathways associated with these receptors. We discuss signaling by the TNF-R family, with an emphasis on how a protypical member of the family, TNF-RI, can induce anti-apoptotic and inflammatory responses in some circumstances or apoptosis in others. As is the case with many membrane receptors in the immune system, the cytoplasmic tails of the TNF-R family members do not contain intrinsic enzymatic activities, but they do contain structural motifs that bind to cytoplasmic signaling molecules and promote the assembly of signaling complexes. One of these structural motifs is called the death domain, and it binds, by homotypic interactions, to death domains in a variety of cytoplasmic signaling/adapter molecules. The cytoplasmic tails of both TNF-RI and Fas contain death domains, and mutation or deletion of this region prevents TNF-RI or Fas molecules from delivering apoptosis-inducing signals. However, death domain interactions are also essential for the anti-apoptotic, proinflammatory signaling pathways. The cytoplasmic signaling molecules that contain death domains and that are involved in TNF-R signaling include TRADD (TNF receptor-associated death domain), FADD (Fas-associated death domain), and RIP (receptor interacting protein). The second type of motif that plays a key role in signaling by several TNF-R family members binds to one of a family of molecules called TNF receptorassociated factors (TRAFs) (see Figure). TNF-RII, LT-βR, and CD40 all bind TRAF proteins. To date, six TRAFs have been identified, and they are called TRAF-1 to TRAF-6. All TRAFs share a C-terminal region of homology called a TRAF domain, which mediates binding to the cytoplasmic domains of TNF-R family members, and TRAFs 2 to 6 have RING and zinc finger motifs that are involved in signaling. Multiple different interactions of death domain proteins and TRAFs are known to occur in the signaling pathways associated with the different TNF-R family members. In the case of TNF-RI, a current model of these interactions illustrates how TNF may lead to either apoptosis or inflammatory responses (see Figure). In this model, the death domain protein TRADD binds directly to the TNF-RI death domain and acts as a pivotal adapter molecule leading to one of two different signaling cascades. In one of these pathways, the death domain protein FADD binds to TRADD, and the aspartyl-directed cysteine protease caspase-8 binds to FADD. Caspase-8 binding initiates the activation of a cascade of caspases that eventually causes apoptosis. In response to Fas ligand binding, Fas mediates apoptosis by a similar pathway, but in this case, FADD binds directly to the cytoplasmic tail of Fas (see Chapter 10, Box 10-2). The alternative proinflammatory and anti-apoptotic pathway involves the binding of TRAF-2 and RIP-1 to TRADD and results in NF-κB-and AP-1dependent gene transcription. Both TRAF-2 and RIP-1 are involved in the activation of IκB kinases, which mediate the initial steps in NF-κB activation (see Chapter 8, Box 8-4). Gene knockout studies suggest that TRAF-5 may be able to substitute for TRAF-2 in NF-κB activation. TRAF-2 also activates the mitogen-activated protein kinase (MAP kinase) cascade that leads to JNK activation, phosphorylation of c-Jun, and formation of the AP-1 transcription factor composed of c-Fos and c-Jun. The combination of NF-κB and AP-1 activation promotes the transcription of a variety of genes involved in inflammation, including endothelial adhesion molecules, cytokines, and chemokines. Furthermore, NF-κB also enhances expression of a family of cellular inhibitors of apoptosis (cIAPs), which block the function of caspases. Therefore, when the proinflammatory pathway is engaged, there is active inhibition of the apoptotic pathway. It is still unclear what determines whether TNF binding to TNFR-1 will activate the inflammatory or apoptotic signaling pathways. Other members of the TNF-R family use TRAF-dependent signaling pathways that lead to NF-κB and AP-1 activation similar to the TNF-RI pathway, but in contrast to TNF-RI, these other receptors directly bind TRAFs to their cytoplasmic tails. TRAF-1 and TRAF-2 interact with TNF-RII, TRAF-2 and TRAF-3 interact with CD40, and TRAF-4 interacts with LT-βR. CD40 delivers activating signals to B cells and macrophages through TRAF binding (see Chapters 9 and 13). TNF-RII, which lacks a death domain, can sometimes deliver apoptotic signals, but the mechanism is not understood. Interestingly, one of the transforming gene products of the Epstein-Barr virus encodes a selfaggregating TRAF domain-containing protein that binds TRAF molecules, and therefore infection by the virus mimics TNF-or CD40-induced signals. Although the IL-1 receptor is not a member of the TNF-R family, many of the biologic effects of IL-1 are similar to the effects of TNF-α because the IL-1 signaling pathway shares biochemical intermediates with the TNF pathway. Binding of IL-1 to the type I IL-1 receptor (IL-1R) leads to the recruitment of an adapter protein, called MyD88, and a serine/threonine kinase called IL-1 receptor-associated kinase (IRAK). IRAK autophosphorylates and dissociates from the complex and subsequently binds TRAF-6. The IRAK-TRAF-6 complex activates both NF-κB and AP-1. This signaling pathway is also used by the Toll-like receptors that are involved in innate immunity (see Chapter 12, Box 12-1). IL-18 also signals by a similar mechanism, and the cytoplasmic regions of the receptors for IL-1 and IL-18 are homologous to each other. BOX 11-2 Cytokine Signaling by Janus Kinases and Signal Transducers and Activators of Transcription One of the best understood mechanisms by which cytokines transduce signals that elicit specific responses in target cells involves enzymes called Janus kinases (JAKs) and transcription factors called signal transducers and activators of transcription (STATs). The JAK/STAT signaling pathways are used by all type I and type II cytokine receptors. Studies of these pathways have revealed direct links between cytokine binding to receptors and transcriptional activation of target genes. The discovery of the JAK/STAT pathways came from biochemical and genetic analyses of interferon (IFN) signaling. The promoter regions of genes responsive to IFNs contain sequences that bind cellular proteins that are phosphorylated upon IFN treatment of the cells. These proteins were shown to activate transcription of cytokine-responsive genes, and they were therefore called STAT proteins. Mutant cell lines were generated that were unresponsive to IFNs. Some of these mutant cell lines were found to lack particular STAT proteins, and introduction of the STATs by gene transfection restored cytokine responsiveness in the cells. This established the essential roles of the STATs in responses to the cytokines. Other mutant cell lines were found to be deficient in one or more related tyrosine kinases, which were called Janus kinases after the two-headed Roman god because of the presence of two kinase domains (only one of which is active). Subsequent studies, including analyses of knockout mice lacking the various STATs and JAKs, have shown that JAK/STAT pathways are involved in responses to many cytokines (see Table). The sequence of events in the JAK/STAT signaling pathways is now well defined (see Figure). Inactive JAK enzymes are loosely attached to the cytoplasmic domains of type I and type II cytokine receptors. When two receptor molecules are brought together by binding of a cytokine molecule, the receptor-associated JAKs become active through transphosphorylation, and they phosphorylate tyrosine residues in the cytoplasmic portions of the clustered receptors. Some of these phosphotyrosine moieties of the receptors are recognized by Src homology 2 (SH2) domains of monomeric cytosolic STAT proteins, which become attached to the receptors. The STAT proteins are then phosphorylated by the receptor-associated JAK kinases. The SH2 domain of one STAT protein is able to bind to the phosphotyrosine residues of another STAT protein. As a result, two STAT proteins bind to each other and dissociate from the receptor. The STAT dimers migrate to the nucleus, where they bind to DNA sequences in the promoter regions of cytokine-responsive genes and activate gene transcription. After each round, new STAT proteins can bind to the cytokine receptor, become phosphorylated, dimerize, and again One intriguing question that arises is, What determines the specificity of responses to many different cytokines, given the limited numbers of JAKs and STATs used by the various cytokine receptors? The likely answer is that unique amino acid sequences in the different cytokine receptors provide the scaffolding for specifically binding, and thereby activating, different combinations of JAKs and STATs. The SH2 domains of different STAT proteins selectively bind to the phosphotyrosine residues and flanking regions of different cytokine receptors. This is largely responsible for the activation of particular STATs by various cytokine receptors and therefore for the specificity of cytokine signaling. In addition, cytokines activate signaling pathways and transcription factors other than STATs. For instance, the IL-2 receptor β chain activates Ras-dependent MAP kinase pathways that may be involved in gene transcription and growth stimulation. Other cytokine receptors may similarly activate other signaling pathways in concert with the JAK/STAT pathways to elicit biologic responses to the cytokines. JAK/STAT Involved cytokines* Phenotype of knockout mice STAT1 IFN-α/β, IFN-γ Defect in innate immunity; no reponse to IFNs STAT2 IFN-α/β Defective immunity to viruses STAT3 IL-6, IL-10 Embryonic lethal STAT4 IL-12 Defect in TH1 development, IFN-g production STAT5a Prolactin Lactation defect STAT5b Growth hormone Dwarfism STAT5a and IL-2, IL-7, IL-9 (in addition Lactation defect, dwarfism, and defective T cell STAT5b to above) proliferation in response to IL-2 STAT6 IL-4 Defect in TH2 development, IL-4-dependent Ig isotypes JAK1 IFN-α/β, IFN-γ, cytokines using γc and gp130 (e.g., IL-2, IL-4, IL-6) Perinatal lethal; defective innate immunity, possible defect in neuronal viability JAK2 Epo, IL-3, IFN-γ Embryonic lethal, hematopoietic failure JAK3 Cytokines using γc chain (IL-7, IL-2, IL-4) Defect in T cell maturation Tyk2 IFN-α/β, IFN-γ, IL-12, IL-10, others Defective IL-12 response of NK cells, defective immunity to viruses *Selected examples of involved cytokines are shown. Several mechanisms of negative regulation of JAK/STAT pathways have been identified. Proteins called suppressors of cytokine signaling (SOCS) are a family of STAT pathway inhibitors. Members of this family are identified by the presence of an SH2 domain and a conserved 40-amino acid C-terminal region called a SOCS box. Eight different SOCS proteins have been identified; in addition, other SOCS box-containing proteins that do not have SH2 domains also exist. SOCS proteins inhibit cytokine actions by binding to phosphotyrosines in the cytoplasmic regions of cytokine receptors, or by binding to and inhibiting the kinase activity of JAKs. The SOCS box appears to be involved in targeting associated proteins, such as JAKs, for proteasomal degradation. SOCS gene knockout mice have begun to provide information on the physiologic role of this family. SOCS-1 knockout mice, for example, die at 3 weeks of age because of excessive actions of IFN-γ, and the phenotype can be corrected by crossing the mice with IFN-γ knockout mice. Other knockout mice studies suggest that SOCS-2 inhibits signaling by the receptors for growth hormone and insulin-like growth factor, whereas SOCS-3 regulates IL-6 signaling. The expression of SOCS proteins is induced by the same cytokine stimuli that activate JAK/STAT pathways, and thus they are negative feedback inhibitors. Other inhibitors of cytokine signaling include tyrosine phosphatases, such as SHP-1, which can dephosphorylate and therefore deactivate JAK molecules, and members of the protein inhibitors of activated STAT (PIAS) family, which bind phosphorylated STAT proteins and prevent their interaction with DNA. Table 11-4. Cytokines of Adaptive Immunity Cytokine Principal cell source Principal cell targets and biologic effects Interleukin-2 (IL-2) 14-17 kD T cells T cells: proliferation, increased cytokine synthesis; potentiates Fas-mediated apoptosis NK cells: proliferation, activation B cells: proliferation, antibody synthesis (in vitro) Interleukin-4 (IL-4) 18 kD CD4+ T cells (TH2), B cells: isotype switching to IgE T cells: mast cells TH2 differentiation, proliferation Macrophages: inhibition of IFN-γmediated activation Mast cells: proliferation (in vitro) Interleukin-5 (IL-5) 45-50 kD; homodimer of 20kD subunits CD4+ T cells (TH2) Eosinophils: activation, increased production B cells: proliferation, IgA production Interferon-γ(IFN-γ) 50 kD (glycosylated); homodimer of 21-to 24-kD subunits T cells (TH1, CD8+T Macrophages: activation (increased cells), NK cells microbicidal functions) B cells: isotype switching to opsonizing and complement-fixing IgG subclassesT cells: TH1 differentiation Various cells: increased expression of class I and class II MHC molecules, increased antigen processing and presentation to T cells Transforming growth factorβ(TGF-β) Size 25 kD; homodimer of 12.5-kD T cells, subunits macrophages, other cell types T cells: inhibition of proliferation and effector functions B cells: inhibition of proliferation; IgA production Macrophages: inhibition Lymphotoxin (LT) 21-24 kD; secreted as homotrimer or associated with LTβ2 on the cell membrane T cells Recruitment and activation of neutrophils Lymphoid organogenesis Interleukin-13 (IL- 15 kD 13) CD4+ T cells (TH2) B cells: isotype switching to IgE Epithelial cells: increased mucus production Macrophages: inhibition page Table 11-5. Hematopoietic Cytokines Cytokine Size Principal cell sources Principal cell targets Principal cell populations induced Stem cell factor (c- 24 kD Kit ligand) Bone marrow stromal cells Pluripotent stem cells All Interleukin-7 (IL-7) 25 kD Fibroblasts, bone marrow Immature lymphoid stromal cells progenitors B and T lymphocytes Interleukin-3 (IL-3) 20-26 kD T cells All Immature progenitors Granulocytemonocyte CSF (GM-CSF) 18-22 kD T cells, macrophages, endothelial cells, fibroblasts Immature and committed Granulocytes and progenitors, mature monocytes, macrophages macrophage activity Monocyte CSF (M- Dimer of 70-90 Macrophages, endothelial Committed progenitors CSF) kD; 40-kD cells, bone marrow cells, subunits fibroblasts Monocytes Granulocyte CSF (G-CSF) Granulocytes 19 kD Macrophages, fibroblasts, Committed progenitors endothelial cells Abbreviation: CSF, colony-stimulating factor.