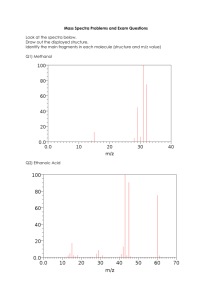
EXPERIMENT 10 – Mass Spectroscopy Problems
advertisement

CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan EXPERIMENT 10 – Mass Spectroscopy Structural Determination Of Compounds . Introduction - In mass spectrometry, a substance is bombarded with an electron beam having sufficient energy to fragment the molecule. The positive fragments which are produced (cations and radical cations) are accelerated in a vacuum through a magnetic field and are sorted on the basis of mass-to-charge ratio. Since the bulk of the ions produced in the mass spectrometer carry a unit positive charge, the value m/e is equivalent to the molecular weight of the fragment. The analysis of mass spectroscopy information involves the reassembling of fragments, working backwards to generate the original molecule. A schematic representation of a mass spectrometer is shown below: A very low concentration of sample molecules is allowed to leak into the ionization chamber (which is under a very high vacuum) where they are bombarded by a high-energy electron beam. The molecules fragment and the positive ions produced are accelerated through a charged array into an analyzing tube. The path of the charged molecules is bent by an applied magnetic field. Ions having low mass (low momentum) will be deflected most by this field and will collide with the walls of the analyzer. Likewise, high momentum ions will not be deflected enough and will also collide with the analyzer wall. Ions having the proper mass-to-charge ratio, however, will follow the path of the analyzer, exit through the slit and collide with the Collector. This generates an electric current, which is then amplified and detected. By varying the strength of the magnetic field, the mass-to-charge ratio, which is analyzed, can be continuously varied. The output of the mass spectrometer shows a plot of relative intensity vs. the mass-to-charge ratio (m/e). The most intense peak in the spectrum is termed the base peak and all others are reported relative to its intensity. The peaks themselves are typically very sharp, and are often simply represented as vertical lines. The process of fragmentation follows simple and predictable chemical pathways and the ions, which are formed, will reflect the most stable cations and radical cations, which that molecule can form. The highest 1 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan molecular weight peak observed in a spectrum will typically represent the parent molecule, minus an electron, and is termed the molecular ion (M+). Generally, small peaks are also observed above the calculated molecular weight due to the natural isotopic abundance of 13C, 2H, etc. Many molecules with especially labile protons do not display molecular ions; an example of this is alcohols, where the highest molecular weight peak occurs at m/e one less than the molecular ion (m-1). Fragments can be identified by their mass-to-charge ratio, but it is often more informative to identify them by the mass which has been lost. That is, loss of a methyl group will generate a peak at m-15; loss of an ethyl, m-29, etc. Click here for a table of common fragments. The mass spectrum of toluene (methyl benzene) is shown below. The spectrum displays a strong molecular ion at m/e = 92, small m+1 and m+2 peaks, a base peak at m/e = 91 and an assortment of minor peaks m/e = 65 and below. The molecular ion, again, represents loss of an electron and the peaks above the molecular ion are due to isotopic abundance. The base peak in toluene is due to loss of a hydrogen atom to form the relatively stable benzyl cation. This is thought to undergo rearrangement to form the very stable tropylium cation, and this strong peak at m/e = 91 is a hallmark of compounds containing a benzyl unit. The minor peak at m/e = 65 represents loss of neutral acetylene from the tropylium ion and the minor peaks below this arise from more complex fragmentation. Common Mass Spectrum Fragments 2 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan Mass Spectrometry - Functional Groups Alkanes: Simple alkanes tend to undergo fragmentation by the initial loss of a methyl group to form a (m-15) species. This carbocation can then undergo stepwise cleavage down the alkyl chain, expelling neutral twocarbon units (ethene). Branched hydrocarbons form more stable secondary and tertiary carbocations, and these peaks will tend to dominate the mass spectrum. Aromatic Hydrocarbons: The fragmentation of the aromatic nucleus is somewhat complex, generating a series of peaks having m/e = 77, 65, 63, etc. While these peaks are difficult to describe in simple terms, they do form a pattern (the "aromatic cluster") that becomes recognizable with experience. If the molecule contains a benzyl unit, the major cleavage will be to generate the benzyl carbocation, which rearranges to form the tropylium ion. Expulsion of acetylene (ethyne) from this generates a characteristic m/e = 65 peak. Aldehydes and Ketones: The predominate cleavage in aldehydes and ketones is loss of one of the side-chains to generate the substituted oxonium ion. This is an extremely favorable cleavage and this ion often represents the base peak in the spectrum. The methyl derivative (CH3C O+) is commonly referred to as the "acylium ion". Another common fragmentation observed in carbonyl compounds (and in nitriles, etc.) involves the expulsion of neutral ethene via a process known as the McLafferty rearrangement, following the general mechanism shown below. Esters, Acids and Amides: As with aldehydes and ketones, the major cleavage observed for these compounds involves expulsion of the "X" group, as shown below, to form the substituted oxonium ion. For carboxylic acids and unsubstituted amides, characteristic peaks at m/e = 45 and 44 are also often observed. 3 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan Alcohols: In addition to losing a proton and hydroxy radical, alcohols tend to lose one of the -alkyl groups (or hydrogens) to form the oxonium ions shown below. For primary alcohols, this generates a peak at m/e = 31; secondary alcohols generate peaks with m/e = 45, 59, 73, etc., according to substitution. Ethers: Following the trend of alcohols, ethers will fragment, often by loss of an alkyl radical, to form a substituted oxonium ion, as shown below for diethyl ether. Halides: Organic halides fragment with simple expulsion of the halogen, as shown below. The molecular ions of chlorine and bromine-containing compounds will show multiple peaks due to the fact that each of these exists as two isotopes in relatively high abundance. Thus for chlorine, the 35Cl/37Cl ratio is roughly 3.08:1 and for bromine, the 79Br/81Br ratio is 1.02:1. The molecular ion of a chlorine-containing compound will have two peaks, separated by two mass units, in the ratio 3:1, and a bromine-containing compound will have two peaks, again separated by two mass units, having approximately equal intensities. 4 CHEM 2423 Mass Spectroscopy- Structural Determination Example (1) – A unknown sample of hydrocarbon with only one oxygen, CxHyO Dr. Pahlavan Mass Spectrum Analysis: MW = 134.18 , C9H10O Degree of unsaturation: D.U. = ( 9 ) – ( 10/2) +1 = 5 ( contains a benzene ring) Parent peak : m/z = 134 Base peak : m/z = 91 Structure: IUPAC Name: 3-pentanone (diethyl ketone) MS Fragments: The spectrum shows a moderate molecular ion peak, and a peak at m-15, strongly suggesting the presence of a labile methyl group. The base peak occurs at m/e = 91, which is highly suggestive of a benzyl fragment, which rearranges to form the tropylium cation. The presence of an intense peak at m/e = 43 is also suggestive of the presence of a methyl ketone which can fragment to form the acylium ion. 5 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan Example(2) – A unknown sample of hydrocarbon with two oxygen atom, CxHyO2 Mass Spectrum Analysis: MW = 100.12, C5H8O2 Degree of unsaturation: D.U. = ( 5 ) – ( 8/2) +1 = 2 Parent peak : m/z = 100 Base peak : m/z = 43 Structure: IUPAC Name: 2-propenyl ethanoate (allyl acetate) MS Fragments: The spectrum shows a small molecular ion peak, and a pair of peaks at m/e = 57 and 58. The peak at m/e = 57 corresponds to loss of m/e = 43, which is the base peak and corresponds to the acylium ion (CH3C O+). The m/e = 57 fragment corresponds to C3H5O, suggesting the original compound was an allyl (-O-CH2CH CH) or methylvinyl (-O-CH CHCH3) ester. Either of these would generate the peak observed at m/e = 41. The peak at m/e = 58 corresponds to the protonated m/e = 57 cation radical. 6 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan Example (3) – A unknown sample of hydrocarbon with halogen, alkylhalide, CxHyX. ( X = Cl, Br, I) Analysis: C7H12Br MW = 171.04 Mass Spectrum Analysis: MW = 171.04, C7H7Br Degree of unsaturation: D.U. = ( 7 ) – ( 7/2) – (1/2) +1 = 4 Parent peak : m/z = 172 Base peak : m/z = 91 Structure: IUPAC Name: bromomethyl benzene (benzyl bromide) MS Fragments: The spectrum shows two small peaks of equal intensity in the molecular ion region, strongly suggesting that the molecule contains bromine (equal concentrations of the 79Br and 81Br isotopes). The base peak represents loss of this bromine to give the peak at m/e = 91, which is highly suggestive of a benzyl fragment, which rearranges to form the tropylium cation. 7 CHEM 2423 Mass Spectroscopy- Structural Determination Dr. Pahlavan EXPERIMENT 10 – Mass Spectroscopy Problems Name _______________________________ Instructor ___________________________ Date ________________________________ Problems Sets – Please Identify the possible structure in each one of the following problems. 1. An organic compound (A) is composed of carbon, hydrogen and nitrogen, with carbon constituting over 60% of the mass. It shows a molecular ion at m/z=112 amu in the mass spectrum. a) Write a plausible Molecular Formula for compound A. b) How many Rings + Double Bonds must be present in compound A? 2. Another compound, B, composed only of carbon, hydrogen and oxygen, also shows a molecular ion at m/z=112 amu. Write a plausible Molecular Formula for compound B, assuming it has three double bonds and no rings. 3. Compound C is composed only of carbon, hydrogen and oxygen, and shows a molecular ion at m/z=180 amu. Carbon accounts for 60% of the molecular mass. a) Write a plausible Molecular Formula for compound C. b) How many Rings + Double Bonds must be present in compound C? 8 CHEM 2423 Mass Spectroscopy- Structural Determination 4. CxHyO 5. CxHyO 6. CxHyO2 7. CxHyX ( X = halogen) 8. CxHyO2 9 Dr. Pahlavan CHEM 2423 Mass Spectroscopy- Structural Determination 9. CxHyO3 10. CxHy 10 Dr. Pahlavan