Nat Genet
advertisement

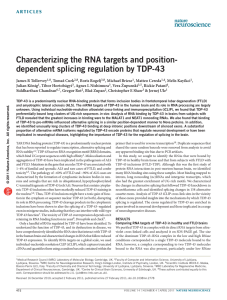
Nat Biotechnol. 2011 Mar 6. [Epub ahead of print] 1.5.2011 דוד Conversion of proteins into biofuels by engineering nitrogen flux. Huo YX, Cho KM, Rivera JG, Monte E, Shen CR, Yan Y, Liao JC. Biofuels are currently produced from carbohydrates and lipids in feedstock. Proteins, in contrast, have not been used to synthesize fuels because of the difficulties of deaminating protein hydrolysates. Here we apply metabolic engineering to generate Escherichia coli that can deaminate protein hydrolysates, enabling the cells to convert proteins to C4 and C5 alcohols at 56% of the theoretical yield. We accomplish this by introducing three exogenous transamination and deamination cycles, which provide an irreversible metabolic force that drives deamination reactions to completion. We show that Saccharomyces cerevisiae, E. coli, Bacillus subtilis and microalgae can be used as protein sources, producing up to 4,035 mg/l of alcohols from biomass containing approx. 22 g/l of amino acids. These results show the feasibility of using proteins for biorefineries, for which high-protein microalgae could be used as a feedstock with a possibility of maximizing algal growth and total CO(2) fixation. PMID: 21378968 Nat Neurosci. 2011 Feb 27. [Epub ahead of print] 1.5.2011 חיים Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Tollervey JR, Curk T, Rogelj B, Briese M, Cereda M, Kayikci M, König J, Hortobágyi T, Nishimura AL, Zupunski V, Patani R, Chandran S, Rot G, Zupan B, Shaw CE, Ule J. TDP-43 is a predominantly nuclear RNA-binding protein that forms inclusion bodies in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). The mRNA targets of TDP-43 in the human brain and its role in RNA processing are largely unknown. Using individual nucleotide-resolution ultraviolet cross-linking and immunoprecipitation (iCLIP), we found that TDP-43 preferentially bound long clusters of UG-rich sequences in vivo. Analysis of RNA binding by TDP-43 in brains from subjects with FTLD revealed that the greatest increases in binding were to the MALAT1 and NEAT1 noncoding RNAs. We also found that binding of TDP-43 to pre-mRNAs influenced alternative splicing in a similar position-dependent manner to Nova proteins. In addition, we identified unusually long clusters of TDP-43 binding at deep intronic positions downstream of silenced exons. A substantial proportion of alternative mRNA isoforms regulated by TDP-43 encode proteins that regulate neuronal development or have been implicated in neurological diseases, highlighting the importance of TDP-43 for the regulation of splicing in the brain. PMID: 21358640 Nature. 2011 Feb 24;470(7335):535-9. 15.5.2011 אור Synaptic potentiation onto habenula neurons in the learned helplessness model of depression Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. The cellular basis of depressive disorders is poorly understood. Recent studies in monkeys indicate that neurons in the lateral habenula (LHb), a nucleus that mediates communication between forebrain and midbrain structures, can increase their activity when an animal fails to receive an expected positive reward or receives a stimulus that predicts aversive conditions (that is, disappointment or anticipation of a negative outcome). LHb neurons project to, and modulate, dopamine-rich regions, such as the ventral tegmental area (VTA), that control reward-seeking behaviour and participate in depressive disorders. Here we show that in two learned helplessness models of depression, excitatory synapses onto LHb neurons projecting to the VTA are potentiated. Synaptic potentiation correlates with an animal's helplessness behaviour and is due to an enhanced presynaptic release probability. Depleting transmitter release by repeated electrical stimulation of LHb afferents, using a protocol that can be effective for patients who are depressed, markedly suppresses synaptic drive onto VTA-projecting LHb neurons in brain slices and can significantly reduce learned helplessness behaviour in rats. Our results indicate that increased presynaptic action onto LHb neurons contributes to the rodent learned helplessness model of depression. PMID: 21350486 Nature. 2011 Jan 27;469(7331):491-7. 15.5.2011 מרינה Comment in News & Views: Nature 469(7331): 474–475 (27 January 2011) A critical role for IGF-II in memory consolidation and enhancement. Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. We report that, in the rat, administering insulin-like growth factor II (IGF-II, also known as IGF2) significantly enhances memory retention and prevents forgetting. Inhibitory avoidance learning leads to an increase in hippocampal expression of IGF-II, which requires the transcription factor CCAAT enhancer binding protein β and is essential for memory consolidation. Furthermore, injections of recombinant IGF-II into the hippocampus after either training or memory retrieval significantly enhance memory retention and prevent forgetting. To be effective, IGF-II needs to be administered within a sensitive period of memory consolidation. IGF-II-dependent memory enhancement requires IGF-II receptors, new protein synthesis, the function of activity-regulated cytoskeletal-associated protein and glycogen-synthase kinase 3 (GSK3). Moreover, it correlates with a significant activation of synaptic GSK3β and increased expression of GluR1 (also known as GRIA1) α-amino-3-hydroxy-5-methyl-4-isoxasolepropionic acid receptor subunits. In hippocampal slices, IGF-II promotes IGF-II receptor-dependent, persistent long-term potentiation after weak synaptic stimulation. Thus, IGF-II may represent a novel target for cognitive enhancement therapies. PMID: 21270887 Nature. 2011 Feb 10;470(7333):221-6. 22.5.2011 אלי Comment in News & Views: Nature 2011 Feb 10;470(7333): 179–181. Functional identification of an aggression locus in the mouse hypothalamus. Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Electrical stimulation of certain hypothalamic regions in cats and rodents can elicit attack behaviour, but the exact location of relevant cells within these regions, their requirement for naturally occurring aggression and their relationship to mating circuits have not been clear. Genetic methods for neural circuit manipulation in mice provide a potentially powerful approach to this problem, but brain-stimulation-evoked aggression has never been demonstrated in this species. Here we show that optogenetic, but not electrical, stimulation of neurons in the ventromedial hypothalamus, ventrolateral subdivision (VMHvl) causes male mice to attack both females and inanimate objects, as well as males. Pharmacogenetic silencing of VMHvl reversibly inhibits inter-male aggression. Immediate early gene analysis and single unit recordings from VMHvl during social interactions reveal overlapping but distinct neuronal subpopulations involved in fighting and mating. Neurons activated during attack are inhibited during mating, suggesting a potential neural substrate for competition between these opponent social behaviours. PMID: 21307935 Dev Cell. 2011 Jan 18;20(1):33-46. 15.5.2011 שירן Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Koch AW, Mathivet T, Larrivée B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvrée K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Bréant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 is an endothelial cell-specific member of the Roundabout axon guidance receptor family. To identify Robo4 binding partners, we performed a protein-protein interaction screen with the Robo4 extracellular domain. We find that Robo4 specifically binds to UNC5B, a vascular Netrin receptor, revealing unexpected interactions between two endothelial guidance receptors. We show that Robo4 maintains vessel integrity by activating UNC5B, which inhibits signaling downstream of vascular endothelial growth factor (VEGF). Function-blocking monoclonal antibodies against Robo4 and UNC5B increase angiogenesis and disrupt vessel integrity. Soluble Robo4 protein inhibits VEGFinduced vessel permeability and rescues barrier defects in Robo4(-/-) mice, but not in mice treated with anti-UNC5B. Thus, Robo4-UNC5B signaling maintains vascular integrity by counteracting VEGF signaling in endothelial cells, identifying a novel function of guidance receptor interactions in the vasculature. PMID: 21238923