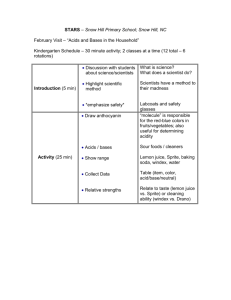
The Marvelous Water Molecule
Water is an important part of all life. The earth’s surface is about 70% water and
all living organisms on earth have water in them. Humans are made up of about 66%
water and chickens are about 75% water. Lettuce is about 90% water. While the amount
of water needed changes from one organism to another, water is one of the basic
requirements for life on earth.
A water molecule is made up of three atoms: two hydrogen atoms and one
oxygen atom. This is why the formula or short-hand for water is written as H2O (two
hydrogens or H2 and one oxygen or O). Hydrogen atoms are smaller than the oxygen and
have a positive charge while the oxygen atom is bigger and has a negative charge.
Because of the shape of the molecule, water has a positive end and a negative end
causing the molecule to act like a magnet!
+
N
+
_
Hydrogen
S
Oxygen
_
These magnet-like positive and negative charges give water special abilities. Molecules
of water can actually stick or bond to each other to form chains just like magnets can
stick to each other. These chains of water molecules create a type of surface film which
allows this water strider to float on water.
1
www.watercampws.uiuc.edu
Photo by:
George I. Bernard/Animals Animals http://encarta.msn.com
Another quality of water is called dissociation. This property of water is responsible for
the formation of acids and bases. In water dissociation, a hydrogen atom breaks away
from the oxygen atom. Here is an example of what happens
+
The chemical reaction is written like this:
H2O
↔
Water equals
H+
OH-
+
one hydrogen ion plus
one hydroxide ion
In pure water there are an equal number of H+ ions and OH- ions. The amount or number
of H+ ions to OH- ions determines the pH of a substance or whether the substance is acid,
base or neutral. This brings us to the pH scale.
The pH scale is a measure of the strengths of acids and bases.
2
www.watercampws.uiuc.edu
Søren Sørensen visting Cornell University in 1924: Courtesy Edgar Fahs Smith Memorial Collection,
Department of Special Collections, University of Pennsylvania Library.
The pH scale was developed by Dr. Sorenson (above) in the early 1900’s. It is believed
that he developed the pH scale to aid the brewing industry. He published his information
about pH in 1920. The letters pH stand for "pondus hydrogenii" or the potential for
hydrogen (H+). The pH scale ranges from 0 to 14, where 0 is the pH of very strong acids
and 14 is the pH of very strong bases. Pure water has a pH of 7 and is a neutral solution,
which means it is neither acidic nor basic and has an equal number of H+ ions and OHions.
Acids
Acids are substances that have hydrogen ions (H+).
The more H+ the stronger the acid.
Acids taste sour.
Vinegar, Citrus Fruits & Coca-Cola are examples of acids.
Any substance with a pH less than 7 is considered an acid.
Bases
Bases are substances that have hydroxide ions (OH-)
The more OH- the stronger the base.
Bases taste bitter and feel slippery, almost soapy (soaps are actually basic!).
Bleach, Ammonia & most cleaning products are examples of bases.
Any substance with a pH greater than 7 is considered a base.
3
www.watercampws.uiuc.edu
The lower the pH of a solution the more acidic it is and the higher the pH of a solution
the more basic it is. A solution of lemon juice or Coca-cola that has a pH of 2 is more
acidic than Seven-up which has a pH of 4. However, lemon juice is not twice as acidic as
Coca-Cola! The lemon juice with a pH of 2 is actually one hundred times more acidic
than the Seven-up with a pH of 4 and has one hundred times more H+ ions! The pH
scale is based on an increase or decrease by a power of ten. Root beer is ten times more
acidic than pure water! Pure water has a pH of 7 and root beer has a pH of 6, the one unit
difference from 7 to 6 means that there is a ten times difference in strength and 10 times
the number of H+ ions. A solution of pH 5 has a 2 unit change with water (from 7 to 5),
so it is 10x10 or one hundred times as acidic as water. A substance like your retainer
cleaner that has a pH of 8 is ten times more basic than water.
Why pH is important
You might be wondering why pH is so important. Our world would be very different if
we did not have acids and bases. Most of the food we eat is acid and our stomach
produces very strong acids. The acids help us digest our food. One of the few foods that
we eat that is basic is beans and we all know the results of that! Our blood has a pH of
about 7.3 which helps our red blood cells to carry oxygen throughout our body! If the pH
of water is too high or basic, minerals can settle out of the water causing our water pipes
to clog and give us low water pressure. If the pH of our water is too low, or acidic,
plumbing fixtures and our hot water heaters can be damaged. Is pH important? Yes, it
is!
4
www.watercampws.uiuc.edu
The Marvelous Water Molecule: Lab
Day One worksheet and Activities
Materials:
Two colors of gum drops per student
Tooth picks
Glue
Cabbage juice
24 cups
12 paintbrushes
cotton Q tips
Substances to be tasted: lemon juice and quinine water
Substances to be touched: soap
pH scale – printable from site
Part One – The Water Molecule
1. Give the students the gum drops and tooth picks and have them construct three water
molecules. Be sure to have the “V” shape form to your molecule.
2. Have the students’ line up their water molecules oxygen to hydrogen to demonstrate
surface film.
Read and discuss the section on acids and bases.
3. Have the students dissociate one of their water molecules. First have them eat the “H”
and then eat the “OH”. Then glue an extra water molecule to the neutral point on the pH
scale and the H+’s on the acid side and OH-’s on the base side.
(Note: Younger students may wish to “act” like water molecules. Groups of three
students can come to the front of the room and form a water molecule. They can separate
and form the two ions. Then, they can rejoin to form the water molecule. This process
can be repeated several times and thus improve understanding of the dissociation.)
4. Have the students taste an example of an acid (lemon juice) and a base (quinine
water) and determine the differences between the taste of the acid and the base. Let the
students feel the base substance (soap) and write your observations below. Have the
5
www.watercampws.uiuc.edu
students read and discuss the characteristics of an acid and a base. Have them choose
three of the characteristics to write on their pH scale paper under characteristics.
5. Have the students discuss and then write next to the number on the pH scale the power
of ten increase in strength, i.e., a pH of 6 is 10 times stronger, a pH of 5 is 10 X 10 or a
100 times stronger, a pH of 4 is 10 X 100 or 1000 times stronger than pure water and so
on.
6. Optional: To emphasize the powers of ten, have the students place one gum drop at
the neutral position , pH of 7, and then count out ten gum drops to put at the pH of 6.
Question how many gum drops should be placed at a pH of 5. Ask what would be the
number of gum drops at a pH of 8. The teacher may wish to give the Power of Ten
worksheet to reinforce this concept.
7. Have the students paint the cabbage juice on the pH scale paper on the grid section so
that it is dry by the next class period. They can also paint the coffee filters.
8. Give the students the “homework” sheet for Monday.
6
www.watercampws.uiuc.edu
The Marvelous Water Molecule Crossword
Across
5. He developed the pH scale.
6. Substances with a pH greater than 7.
7. This atom is larger than the hydrogen atom and has a negative charge.
8. The water molecule is made up of three of these.
9. Substances with a pH less than 7.
Down
1. This ion is associated with bases.
2. The measure of the strengths of acids and bases.
3. This ion is associated with acids.
4. This property of water is responsible for the formation of acids and bases.
7
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Double Puzzle
Unscramble each of the clue words.
Take the letters that appear in
message.
boxes and unscramble them for the final
Answer the questions below:
1. Does an acid taste sour or bitter?
2. Does a base taste sour or bitter?
3. What is the pH range of acids?
_____ to 7
4. What is the pH range of bases?
7 to _____
8
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Powers of Ten Worksheet
Powers of Ten:
Look at the chart below and answer the following questions.
pH and the Powers of Ten
1000000
10000
1000
100
Ten
Power of Ten
100000
10
1
1
2
3
4
more acidic
5
←
6
7
8
pH
9
10
11
12
13
14
→ more basic
1. How much more acidic is an acid with a pH of 6 than water with a pH of 7?
2. How much more basic is a base with a pH of 9 than water with a pH of 7?
3. The line on the right hand side of the graph is not complete. Using your pencil or pen,
draw in a line that completes the graph.
4. Looking at the graph, what shape indicates the neutral section for pH?
5. What is an example of a neutral substance?
6. What is an example of a basic substance?
9
www.watercampws.uiuc.edu
7. How could you use this chart to help determine the strength of an acid or a base?
8. Why is pH important?
Extra credit:
How many times more basic is a base with a pH of 11 when compared to water with a pH
of 7?
Who is Dr. Sorenson?
What is pondus hydrogenii?
10
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Using pH Probes and Creating a Color Scale
pH Lab
Materials needed for a class size of 24:
pH probes/palm pilots or Wide Range pH Test Strips
coffee filters painted with cabbage juice, dried and cut into test strips
12 large pH scales
variety of substances to be tested: lemon juice, vinegar, sprite, Mr. Pibb, pure water,
baking soda solution, household ammonia, quinine water
Q-tips
Part One:
Test each of the samples provided with the pH probe and write their pH on the table
below.
pH
Substance Tested
Lemon juice
Vinegar
Sprite
Mr. Pibb
Pure water
Baking soda Solution
Household Ammonia
Unknown Substance
6. According to the results of the tests, place a small drop of each liquid on the
cabbage juice under the matching number on the pH scale paper. Then label each dot
according to the material used, vinegar, sprite, etc., in the space provided below.
11
www.watercampws.uiuc.edu
Part Two
7. Test the unknown substance with the coffee filter strips.
8. Predict the pH of this unknown by comparing the strip with the color spectrum
on your pH scale paper.
What do you predict the unknown substance to be an acid or a base? Why?
9. Test unknown with pH probe and determine the accuracy of their predictions.
Was your prediction correct?
12
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Using pH Probes and Creating a Color Scale
Follow-up Questions for the pH lab:
1. What is the color range for an acid according to your cabbage juice scale?
2. What is the color range for a base according to your cabbage juice scale?
3. What are the advantages of testing pH with pH probes?
4. What are the advantages of testing pH with an indicator?
5. What word describes the property of water that is responsible for the formation of
acids & bases?
6. What ion or part of a water molecule is associated with an acid?
7. What ion or part of a water molecule is associated with a base?
8. What type of substances would you like to test?
9. Would you prefer to use the pH probes or the cabbage juice to test these
substances? Why?
10. Which part of the water and pH presentation did you like the most?
11. Which part of the water and pH presentation did you like the least?
12. Why do you think that studying pH is important?
13
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Creating Your Own pH Color Scale
pH Lab
Materials needed for a class size of 24:
coffee filters painted with cabbage juice, dried and cut into test strips
12 large pH scales
variety of substances to be tested: lemon juice, vinegar, sprite, Mr. Pibb, pure water,
baking soda solution, household ammonia, quinine water
Q-tips
Cups
Part One:
Below are a variety of household substances and their pH.
Substance Tested
Lemon juice
Vinegar
Sprite
Mr. Pibb
Pure water
Baking soda Solution
Household Ammonia
Unknown Substance
pH
2
3
4
5
7
8
11
6. Using the Q tips as a paint brush, paint the lemon juice in the box beneath the 2 on
the pH chart. Notice the color change. With a new Q tip each time, choose a
substance, find its pH, and paint the appropriate box. You should see a colors emerge
showing you a pH “range of color”.
14
www.watercampws.uiuc.edu
Part Two
10. Test the unknown substance with the coffee filter strips.
11. Predict the pH of this unknown by comparing the strip with the color spectrum
on your pH scale paper.
What do you predict the unknown substance to be an acid or a base? Why?
12. Test unknown with pH probe and determine the accuracy of their predictions.
Was your prediction correct?
15
www.watercampws.uiuc.edu
The Marvelous Water Molecule
Creating Your Own pH Color Scale
Follow-up Questions for the pH lab:
1. What is the color range for an acid according to your cabbage juice scale?
2. What is the color range for a base according to your cabbage juice scale?
3. What are the advantages of testing pH with an indicator?
4. What word describes the property of water that is responsible for the formation
of acids & bases?
5. What ion or part of a water molecule is associated with an acid?
6. What ion or part of a water molecule is associated with a base?
7. What type of substances would you like to test?
8. Which part of the water and pH presentation did you like the most?
9. Which part of the water and pH presentation did you like the least?
10. Why do you think that studying pH is important?
16
www.watercampws.uiuc.edu
pH Reference Scale
Substance
pH
Coke
2.60
Cranapple juice
2.90
Dr. Pepper
3.62
Hi-C
3.70
strawberry cooler
4.05
orange slice
4.17
imit. Mountain Dew
4.20
Hi-C and Sprite
4.24
Surge
4.25
7-Up
4.28
orange Slice
4.29
7-up
4.44
citrus Zephyr soda
4.59
citrus Zephyr soda
4.59
Sunny Delight
4.71
Mr. Pibb
4.90
pickle juice
4.98
hotsauce/aftershave
5.19
mouth wash
5.33
Listerine
5.45
pickle juice
5.55
shampoo
5.62
ibuprofen in water
5.72
root beer
5.79
Pepto Bismol
5.81
Scope
6.34
nailpolish remover
6.50
Ramen broth
6.59
root beer
6.63
soybean milk
6.66
skim milk
6.77
tea
6.79
Pantene shampoo
6.95
plant food
6.99
chocolate milk
7.03
rubbing alcohol
7.17
Pantene shampoo
7.22
Dawn
7.44
ovaltine and milk
7.53
Plax
7.55
409 glass cleaner
7.61
astringent
7.66
Ajax liquid
7.80
green tea
7.90
retainer cleaner
8.04
Denton tap water
8.22
astringent
9.47
detergent in water
9.68
kitchen cleaner
10.90
Liquid Plumr
12.01
Substances used in the lab:
lemon juice – 2, vinegar – 3, sprite – 4,
Mr. Pibb – 5, pure water – 7, baking
soda solution – 8, household ammonia –
11.
17
www.watercampws.uiuc.edu
 0
0