Solubility Curve Worksheet: Chemistry Practice
advertisement

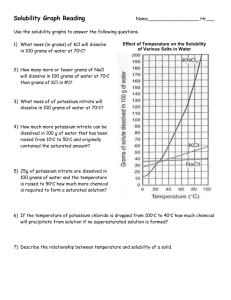
Name: ______________________________ period: __________ Solubility Curve solution of hydrochloric acid; the gas would be released and could be very harmful) 4. Look at the curves and see; how many grams of NH4Cl could dissolve in 100 g of water at 50oC? _________ a. How much (in grams if you used 300 ml (300grams) of water? __________ b. How much if you still used 100 grams of water but heated the water to 80oC? _______________ 5. How many grams of Sodium Nitrate(NaNO3) could dissolve in 100 ml of water at 70oC? _______; at 10oC? _______ 6. A solution of NaNO3 in 100 g water is saturated at 40oC; it is then cooled to 10oC; how much precipitate forms?______ 7. At what temperature do KClO3 and NaCl have the same solubility in water?________ 8. If a 200 g of water is saturated with KCl at 10oC, how much MORE can be added (and still dissolve) if the solution is heated to 70oC? ________ 9. How many grams of NaNO3 are required to saturate 400 grams of water at 10oC? ________ 10. What is the smallest mass of water that will completely dissolve 23 grams of NH4Cl at 40oC? ______ 1. Look at the table above: for most materials how does temperature affect solubility? ___________________ 2. There are 4 compounds listed that don’t follow the general trend: list them: _____________________________ 3. Three compounds you identified above are all gases; the Hydrogen Chloride is soluble enough in water that I have told you to call it hydrochloric acid and your experience is with it as a solution (but that’s why I don’t want you to ever Boil a What mass of water for 100 grams at 40oC? _____ Name: ____ANSWERS___________________ period: __________ Solubility Curve solution of hydrochloric acid; the gas would be released and could be very harmful) 14. Look at the curves and see; how many grams of NH4Cl could dissolve in 100 g of water at 50oC? __50g NH4Cl___ a. How much (in grams if you used 300 ml (300grams) of water? ___150 g__(3 times as much as for 100 g H2O) b. How much if you still used 100 grams of water but heated the water to 80oC? ___65 g_NH4Cl___ 15. How many grams of Sodium Nitrate(NaNO3) could dissolve in 100 ml of water at 70oC? __135 g_; at 10oC? __79 g_ 16. A solution of NaNO3 in 100 g water is saturated at 40oC; it is then cooled to 10oC; how much precipitate forms? __~23 g_ 17. At what temperature do KClO3 and NaCl have the same solubility in water?__74oC____ 18. If a 200 g of water is saturated with KCl at 10oC, how much MORE can be added (and still dissolve) if the solution is heated to 70oC? __38 g (~19 g x 2)____ 19. How many grams of NaNO3 are required to saturate 400 grams of water at 10oC? __320 g ( 80 g x 4)______ 20. What is the smallest mass of water that will completely dissolve 23 grams of NH4Cl at 40oC? __50 ml____ 11. Look at the table above: for most materials how does temperature affect solubility? __as temp increases so does solubility___ 12. There are 4 compounds listed that don’t follow the general trend: list them: __SO2, NH3, HCl and Na2SO4____ 13. Three compounds you identified above are all gases; the Hydrogen Chloride is soluble enough in water that I have told you to call it hydrochloric acid and your experience is with it as a solution (but that’s why I don’t want you to ever Boil a a. What mass of water for 100 grams at 40oC? __217.4 g_ 46 g NH4Cl / 100 g H2O = 100 g NH4Cl / ? g H2O ? = 214.7