Aflatoxin Enzyme Immunoassay Test Kit
advertisement

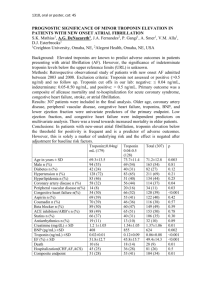
TROPONIN I Immunoassay Test Kit Introduction Cardiac Troponin I (cTnI) is a cardiac muscle protein with a molecular weight of 22.5 kilodaltons. In the heart it forms a protein complex together with Troponin T and Troponin C. The Troponin I complex is broken up following myocardial damage, and the individual protein components are released into the bloodstream. Although Troponin I is also found in skeletal muscles, this form differs from cTnI in its amino acid composition. This distinction allows the two forms of Troponin I to be distinguished immunologically and thereby ensures an accurate test assay. Cardiac Troponin I is released to blood circulation soon after the onset of cardiac damage. Approximately 4 to 6 hours following an acute myocardial infarction (AMI), a detectable level of cTnI can be detected with our immunochromatographic test. While the normal serum level of cTnI is below 0.06ng/ml, levels as high as 100-1300ng/ml in some AMI patients. The Troponin I Enzyme Immunoassay Test Kit is a quantitative test that can be used to determine the concentrations of Troponin I in serum samples. It can be used together with other diagnostic methods to assess cardiac damage caused by AMI. Reagents Materials supplied with the kit Anti-Troponin I coated microtiter wells Reference standards: 0, 1.5, 5, 20, 50, 100ng/mL (free form) Enzyme Conjugate Reagent TMB substrate Stop solution (2N HCl) Materials needed but not supplied with the kit: Precision pipettes for the following measurements: 50, 100, and 200L Repeating pipette (optional) Disposable pipette tips Distilled water Glass tubes or microcentrifuge tubes for mixing solutions Paper towel or other absorbent paper Graph paper or computer graphing program Microtiter plate reader capable of measuring at 450 nm Specimen Collection and Preparation Collect blood in a tube without anticoagulant and allow clotting. Since cardiac proteins are relatively unstable, it is recommended that fresh samples be used as soon as possible to collect critical patient information. Heat inactivation of samples may lead to hemolytic or protein denaturation and therefore should be avoided. Storage and Handling Store the Troponin EIA Kit at room temperature The shelf life of the test is indicated on the test pouch Wear disposable glove while handling specimens and thoroughly wash hands afterwards. All patient samples should be handled as if they are potentially infectious. If serum or plasma samples have been stored in the refrigerator, allow them to return to room temperature before testing. Storage Conditions All components of the kit should be stored at 4C when not in use. Keep the microtiter wells in a sealed bag with desiccant. Instrumentation A microtiter well reader with an optical density range of 0 to 3 OD or greater, at 450nm wavelength, is ideal for this kit. Assay Procedure 1. Bring all components of the kit to room temperature (25°C) before use. 2. Break off the proper number of antibody coated wells (at least two for each of the Eight standards plus two or more for each of the samples). 3. Dispense 100uL of standards, specimens and controls into appropriate wells. 4. Dispense 100uL of Enzyme Conjugate Reagent into each well. 5. Thoroughly mix for 30 seconds. It is very important to mix completely. 6. Incubate for 90 minutes at 37 0 C. 7. Remove the incubation mixture by flicking plate contents into a waste container. 9. Rinse and flick the microtiter wells 5 times with distilled or deionized water. (Please do not use tap water). 10. Strike the wells sharply onto absorbent paper or paper towels to remove all residual water droplets. 11. Break off 2 or more microtiter wells for “blanks” 12. With a very clean repeating pipette (washed well with deionized water), add 100uL per well of TMB Reagent. 13. Incubate at room temperature for 20 minutes. 14. Then with repeating pipette, add 100uL of Stopping Solution (2N HCl) to each well. 15. Read the OD450 with a microtiter plate reader. Interpretation of Results 1. Calculate the average optical density value (OD450) for all sets of the reference standards, unknown samples and blanks. 2. Subtract the average blank value from the average values of the reference standards and unknown samples. 3. Produce a standard curve for the reference standards by plotting their Troponin I concentrations in ng/mL on the X-axis and their corresponding OD450 values on the Y-axis. 4. Use the standard curve to determine the Troponin I concentration in the unknown sample.