Intensive Care Unit Research Coordinator Funding Application
advertisement

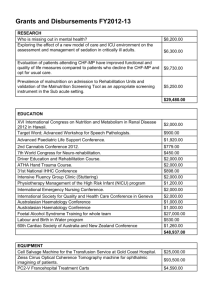
Intensive Care Unit Research Coordinator Funding Application Executive Summary Overview This document is in support of a request for sustainable funding for the employment of *** FTE of Research Coordinator (RC) in the ICU of *** hospital. The estimated cost of this is ***. Research involving patients in Intensive Care Units is unique as it involves unconscious patients in a complex medical environment. Within Australia and New Zealand an effective model of specialist research coordinators working closely with principal investigators has developed to address the challenges of performing clinical trials in this area. This model is widely acknowledged to be both effective and efficient and has been widely copied elsewhere. The importance of research has been recognised by the organisations that accredit hospitals and specialist training. Participation in research is a requirement of hospital accreditation and also for the accreditation of Intensive Care Units to conduct specialist training. The EQuIP5 Mandatory Functions, Standards and Criteria of the Australian Council on Healthcare Standards (ACHS), mandates that clinical research is encouraged and is subject to adequate governance, protection of patient safety and management of organizational risk. Fulfilling these requirements are key responsibilities of the RC. The availability of a funded research coordinator is now a condition of accreditation of ICUs for specialist training by the College of Intensive Care Medicine. The Australian Council on Health Care Standards, Australian College of Critical Care Nurses, Australian Nursing and Midwifery Council, and the Joint Faculty of Intensive Care Medicine all have formal policies that recognise the critical importance of research activities. Track record of Intensive Care Research The Intensive Care community in Australia and New Zealand has a strong track record in the design and execution of high-quality multicentre randomised controlled trials. Clinical trials of this nature provide the highest form of evidence to inform changes in clinical practice. Many of the trials conducted have resulted in both better outcomes for patients and reductions in the cost of care through reduced use of expensive but ultimately ineffective treatments. The ANZICS-CTG is a collaborative group of intensive care researchers in Australia and New Zealand that has an unparalleled record in the conduct of large pragmatic multi-centre non-commercial research. The CTG is funded by its 71 member units and has in excess of 550 clinicians subscribed to its mailing lists. The Intensive Care Research Coordinators Interest Group (IRCIG) is closely allied to the ANZICS-CTG and exists to support and promote the role of Research Coordinators within Australia and New Zealand. To date the CTG has received in excess of Au$55m in competitive funding and included over 44,000 patients in clinical trials. The major source of funding for these clinical trials is government grants through the National Health and Medical Research Council (NHMRC) of Australia and the Health Research Council of New Zealand (HRC). The outcomes of these trials include more than 88 peer reviewed publications and some significant changes to practice guidelines. Through the efforts of the ANZICS–CTG and IRCIG the ICU research infrastructure has evolved in to an efficient organization consisting of Principal Investigators (predominantly ICU Specialists) and Research Coordinators at each site. The infrastructure, experience and networks developed through the conduct of CTG trials is an invaluable resource for member units allowing participation in other research projects and also in many cases supporting the conduct of quality assurance, teaching and audit activities in addition to formal research. The role of the RC is complex and broad and includes managing many aspects of individual study conduct including ethics and regulatory approval, recruiting and consenting patients and data collection. Indirect benefits of having an RC employed as part of the ICU team includes provision of expert advice for data collection for non-research activities (such as audit and databases) and many RCs have a significant educational role with respect to translation of research into practice. This structure is highly efficient, both in terms of overall cost and in the timely completion of studies. Benefits of research Direct benefits ICU research has the potential to both improve patient outcomes and substantially reduce costs, including direct ICU treatment costs and costs incurred throughout the health service for the rehabilitation of patients following their critical illness. Several recently completed large multi-centre trials conducted by the ANZICS-CTG have already resulted in large savings to the public health system. Many of these trials studied the effectiveness of treatments that were becoming de facto standards of care despite increased costs and little evidence of clinical benefits. Although the primary aim of these studies was to assess clinical effectiveness, some conclusions about comparable cost effectiveness can be made, particularly when a new treatment that is steadily becoming standard care because of theoretical benefits is shown to be ineffective in a robust trial. Some examples of these trials include; 1. NICE-SUGAR – A trial comparing the use of intensive insulin treatment with standard care. IIT was shown to not only be more expensive but also had higher patient mortality than standard care. This landmark trial resulted in the removal of the recommendation to use IIT from many international guidelines. 2. DECRA – A study comparing the use of surgical treatment of severe head injuries with non-surgical treatment. DECRA showed that the surgically treated patients did much worse than the medically treated ones. This study has resulted in significantly reduced usage of surgical decompression and is estimated that the savings from the results of this study on the management of traumatic brain injury will save the Australian health sector $100 million p.a. 3. RENAL – demonstrated that no benefits were found by using a higher dose of renal replacement therapy (RRT) – saving up to $200 per day for RRT use. As 5% of all ICU patients require RRT this represents a very significant saving to the sector. 4. Early PN – a study comparing early intravenous nutrition (PN) with standard care that demonstrated no benefits to the more costly PN. 5. CHEST – demonstrated that a synthetic intravenous colloid was no better than normal saline as a resuscitation fluid for intensive care patients yet costs 5-6 times more. The funding for these investigator initiated or ‘public good’ studies is highly competitive and often only covers the direct costs of conducting a specific study – i.e. the time required for patient enrollment, study procedures and data collection. The time required for ethical and institutional approval and governance processes, study startup and staff education is not usually included. The design phase for these large multi-centre studies can take many years and involve observational and pilot studies that although essential for successfully obtaining funding for the large trial are often not themselves financed. In addition to the long term operational benefits and patient centered outcomes achieved by translating the results of these landmark trials into clinical practice, ICUs involved in research can also benefit from the direct operational savings achieved through the provision of free or subsidized patient treatment as part of clinical trials. Recent trials have provided intravenous fluid and sedative drugs, both widely used treatments in ICU, free of charge for patients enrolled in studies. Indirect benefits There are numerous additional benefits of having an active and successful research program in intensive care, including; 1. It is a requirement for recognition for training by the College of Intensive Care Medicine. 2. An active research program fosters a culture of evidence based practice within ICU. 3. ICU’s that are active in research are seen as an attractive place to work for both medical and nursing staff, and senior medical staff? 4. Familiarity with research procedures and data collection techniques facilitates the conduct of non-research audit. 5. Single site studies – Although not designed to change practice, single site studies are a vital component of all research programs. Benefits include development work for proposed larger multicentre studies, individual unit research to answer specific departmental research questions and projects for medical and nursing staff. The latter includes projects that clinical staff undertake as part of higher study or specialist training. Many single centre studies, including those done by trainee medical staff and nurses often require assistance from the Research Coordinators (RCs) yet are not funded. Commercial research This is predominantly pharmaceutical company or medical device company supported research. The amount of commercial research performed in intensive care is limited with many sites reporting significant reductions in the revenue from such studies. Current structure of research in this ICU The ICU in this hospital has been involved in clinical research for *** years. There are currently *** Research Coordinators sharing ***FTE and *** of the ICU specialists are involved in research. Threats to research The single biggest threat to the ongoing viability of research in this ICU is financial sustainability which impacts on the recruitment and retention of Research Coordinators. This application is for permanent ongoing funding for *** FTE of Research Coordinator This proposed enhanced funding model will ensure the continued/permanent employment of the ICU RC and the continued provision of research within this unit. It will also provide us the benefit of undertaking the most appropriate studies for our ICU and our patients rather than having to do studies based on financial reward. By having a permanent RC appointed to this unit, we ensure the retention of specialist knowledge and skill rather than hiring and firing staff on a project by project basis. It also ensures that vital unit specific research can be undertaken as desired. By undertaking research we are improving patient outcomes and delivering savings through the implementation of proven cost-effective interventions.