Chapter 13: NMR
advertisement
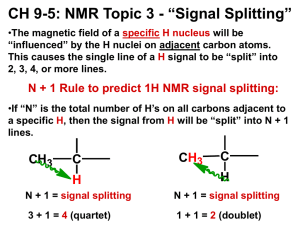
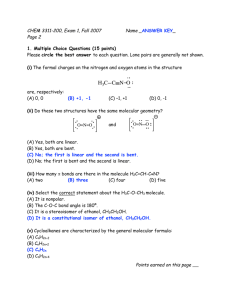
Chemistry 241 Chapter 13: Nuclear Magnetic Resonance (NMR) Spectroscopy HW: 5, 10, 11, 17, 20, 25, 32, 37, 43, 45, 46, 48, 50, 54, 56, 59, 61, 71 I. How it works (not emphasized, but may help understanding of topic) A. Molecules subject to applied magnetic field, then allowed to relax B. electromagnetic signals released upon relaxing give info about H’s on molecule The printout A. vertical axis records intensity – see integration, below B. horizontal axis is chemical shift relative to TMS (tetramethylsilane) 1. δ (ppm) units for chem shift 2. TMS at zero (shielded/upfield) 3. 10 (deca/deshielded/downfield) The emission signal A. Chemical shift 1. look up on table such as on p580 or in appendix VI 2. values are approx. and cumulative (but not additive) a. ie, an H on a carbon with an ether (Chem shift ~3.3) and a Cl (chem shift ~3-4) would have a net chem shift ~4-5 3. chemical shift found in center of split signal 4. all sp3 H’s on same carbon are chemically equivalent 5. 2 H’s on same C in double bond may not be equivalent 6. chemically equivalent H’s have same chemical shift and do not split each other (see below) 7. exercise: how different many signals in... approx. chem shift of.. 8. no convention on which is “a,” “b,” “c,” etc. II. III. H H H H H H H HBr C H H H H H Cl H3C CH3 H2 C H3C H C H2 H2 C C H2 CH3 B. Integration 1. the area under each signal corresponds to #H’s: integration 2. ratio of areas for 2 signals = ratio of # H’s a. ie, a one to one ratio for two signals implies same number of H’s in each signal, but actual number could be 1:1, 2:2, 3:3, etc. b. a 1:1.5 area ratio means 2H:3H or 4H:6H, etc. 3. add up total height of all integrations, divide by the total number of H’s. This is a conversion factor to go from height to # of H’s 4. 3H’s could be a CH3 or 3 equivalent CH’s (unlikely) but could not be a CH2 and a CH (why not?) 5. exercise: what would be integration ratio in (one:?) 1 CH3-CH2-CH2-OCH3 CH3-CH=CH2 CH2Br-CH (CH3)2 C. Splitting 1. each signal may be split by adjacent H’s 2. n+1 rule, where n+1 = #peaks in signal 3. n is the number of adjacent equivalent H’s example: CH3-CHBr2 has CH3 next to CH: a doublet CH next to CH3: a quartet 4. note: split depends on adjacent H’s 5. if non equivalent, then multiply n+1’s example: CH3-CH2-CH2Br center CH2 next to CH3 and CH2Br- not equivalent! center CH2 split into triplet by CH2Br AND then further split into quartet by CH3 → a “twelve-plet” which is simply called a multiplet 6. Splitting pattern intensity by Pascal’s triangle # adjacent equivalent H’s 0 1 2 3 4 #multiple peaks in signal (n+1) singlet doublet triplet quartet quintet relative peak intensities 1 1:1 1:2:1 1:3:3:1 1:4:6:4:1 7. exercise what is splitting pattern for each of these signals? Br a (CH3)3-C-CH2-CH3 IV. V. CH2Br-OCH-(CH3)2 b d c e D. Coupling 1. only useful on higher end machines, otherwise difficult to discern 2. distance in hertz between adjacent split signals = coupling constant OH’s and NH’s A. H-bonding usually means these signals are broad, not split, nor do they split others 13 C NMR 2 VI. A. Tells # of equivalent C’s B. Chemical shift: again related to chemical environment of C’s C. Proton coupled: can get number of H’s on a C D. Proton decoupled: # signals = # equivalent C’s Even more complicated (intro only- not for memorization) A. 2-D NMR: COESY, NOESY, etc B. MRI Objectives Knowledge Define new terms: upfield/downfield, shielded/deshielded, chemical shift, TMS, delta (ppm), spin splitting, n + 1 rule Comprehension Understand meaning of splitting pattern (n +1 rule regarding neighboring H’s), integration, and chemical shift (given chemical shift tables) Application Predict likely NMR spectra of a given molecule (chemical shift, splitting, integration) for both H-NMR and 13C-NMR Analysis Interpret NMR spectra to deduce molecular structure 3