NPH_3977_sm_FigS1-S4-TableS1
advertisement

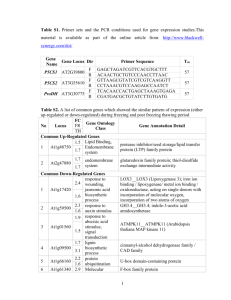
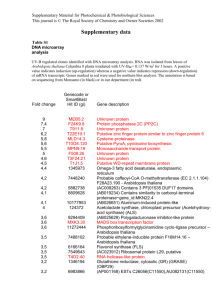
Supporting Information Fig. S1 Frequency distribution of leaf element concentration across the 31 Arabidopsis accessions used in this study for (a) Ca, (b) Mg and (c) Zn. Weight normalised leaf element concentration data for these accessions were obtained from the iHUB consortium website (http://www.ionomicshub.org) and normalised using the REML method proposed by Broadley et al. (2010). These values were partitioned across equal bins from their minimum to maximum values for Ca (39,737-51,223 mg kg-1 DW), Mg (12,132–16,721 mg kg-1 DW) and Zn (24.9–111.2 mg kg-1 DW) and the number of accessions falling within each concentration range is displayed on the y-axis. Fig. S2 Distribution of Pearson correlation coefficients (r values) for each transcript (with log2 expression value ≥ 3.5 in at least one sample) each specific element (Ca, Mg and Zn). Each correlation approaches a normal distribution, with no obvious skewed distribution. Arrows indicate threshold applied to indicate significantly correlated transcripts (r < -0.3; r > 0.3). Note outermost bins represent values ≤ -0.5 and ≥ 0.5, respectively. Calcium (Ca) (a) (b) (c) Magnesium (Mg) r = 0.220 P = 0.234 r = -0.027 P = 0.884 Zinc (Zn) r = -0.039 P = 0.833 r = 0.285 P = 0.120 r = 0.395 P = 0.028 r = 0.100 P = 0.594 r = -0.010 P = 0.957 r = 0.251 P = 0.173 r = -0.197 P = 0.288 r = -0.048 P = 0.797 r = -0.397 P = 0.027 r = 0.032 P = 0.863 r = -0.003 P = 0.989 r = 0.384 P = 0.033 r = 0.340 P = 0.061 (d) r = -0.369 P = 0.041 (e) (f) (g) (h) (i) (j) (k) r = 0.378 P = 0.036 r = -0.091 P = 0.628 r = 0.168 P = 0.367 r = 0.563 P = 0.001 r = 0.066 P = 0.724 r = -0.013 P = 0.945 r = -0.367 P = 0.042 r = -0.023 P = 0.903 r = -0.392 P = 0.029 r = 0.081 P = 0.666 r = -0.318 P = 0.081 r = -0.106 P = 0.569 r = -0.214 P = 0.248 r = 0.143 P = 0.444 r = 0.321 P = 0.023 Fig. S3 Scatter plots generated with the population correlation filter referred to in the main text. Line of best fit, Pearson correlation coefficient (r) and P-value (P) shown for each graph for the following genes: (a) AtCAX1 (At2g38170, 267093_at), (b) AtMRS2-1 (At1g16010, 261795_at), (c) AttDT (At5g47560, 248756_at), (d) AtVHA-a3 (At4g39080, 252932_at), (e) AtKUP5 (At4g33530, 253330_at), (f) AtCCX4 (At1g54115, 263154_at), (g) AtTPC1 (At4g03560, 255380_at), (h) AtMTP5/AtMTPc2 (At3g12100, 256272_at), (i) AtKUP3 (At3g02050, 258860_at), (j) AtMCA1 (At4g35920, 253109_at), (k) AtMTP1 (At2g46800, 266718_at). Ca and Mg correlations for AtCAX1 and AtMRS2-1, respectively, are excluded from this figure as they are presented in the main body. Fig. S4 The expressed probesets (log2 expression value ≥ 3.5) from the microarrays where used for average linkage clustering. The distance between two samples corresponds to 1Spearman correlation coefficient over the whole array. The divergence of the Frankfurt-2 accession (name highlighted in red), which was also seen in pairwise correlation plot matrices, meant it was removed from all further correlation analyses. Accessions used in the population correlation filter are: Achkarren-1, Bayreuth-0, Blanes-5, C24, Caen-0, Canary Islands-0, Cape Verde Islands (Cvi-0), CIBC10, Columbia-0, Drahonin-1, Enkheim-T, Erlangen-0, Estland, Frankfurt-2, HR-5, Isenburg-0, Kindalville-0, Landsberg erecta (Ler-1), Limburg-2:1, Moscow-0, Neuweilnau-1, NFE1, Niederzenz-1, Noordwijk-1, Oldenburg-1, Ovelgoenne-0, San Eleno-0, San Feliu-2e, Shahdara, Tabor-0, Umkirch-3, Vancouver-0. Table S1 Studies incorporating natural Arabidopsis accessions to investigate elemental accumulation phenotypes or implicate genes in these processes Phenotype Aluminium (Al) Technique(s) used QTL (Col-0 × Ler) Reference Hoekenga OA, Maron LG, Pineros MA, Cancado GM, Shaff J, Kobayashi Y, Ryan PR, Dong B, Delhaize E, Sasaki T et al. 2006. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proceedings of the National Academy of Sciences, USA 103: 9738-9743. Kobayashi Y, Koyama H. 2002. QTL analysis of Al tolerance in recombinant inbred lines of Arabidopsis thaliana. Plant & Cellular Physiology 43: 1526-1533. Cadmium (Cd) Hoekenga OA, Vision TJ, Shaff JE, Monforte AJ, Lee GP, Howell SH, Kochian LV. 2003. Identification and characterization of aluminum tolerance loci in Arabidopsis (Landsberg erecta × Columbia) by quantitative trait locus mapping. A physiologically simple but genetically complex trait. Plant Physiology 132: 936-948. QTL (A. halleri × A. Courbot M, Willems G, Motte P, Arvidsson S, Roosens N, Saumitou-Laprade P, lyrata) Verbruggen N. 2007. A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4, a gene encoding a heavy metal ATPase. Plant Physiology 144: 1052-1065. Willems G, Frérot H, Gennen J, Salis P, Saumitou-Laprade P, Verbruggen N. 2010. Quantitative trait loci analysis of mineral element concentrations in an Arabidopsis halleri × Arabidopsis lyrata petraea F2 progeny grown on cadmium-contaminated soil. New Phytologist 187: 368-379. Cationic mineral content (Ca, QTL (Ler × Cvi) Vreugdenhil D, Aarts MGM, Koornneef M, Nelissen H, Ernst WHO. 2004. Natural Fe, K, Mg, Mn, Na, Zn) variation and QTL analysis for cationic mineral content in seeds of Arabidopsis thaliana. Plant, Cell & Environment 27: 828-839. Cell specific calcium Cell specific elemental Conn, S Gilliham, M Schreiber, A Baumann, U Moller, I Cheng, N-H Stancombe, MA accumulation and microarray profiling Athman, A Hirschi, K Webb, AAR et al. 2011a. Cell-specific vacuolar calcium storage mediated by AtCAX1 regulates apoplastic calcium concentration, gas exchange and plant productivity. Plant Cell 23: 240-257. Cell specific magnesium Cell specific elemental Conn, S Conn, V Tyerman, S Kaiser, B Leigh, R Gilliham, M. 2011b. Arabidopsis accumulation and microarray profiling magnesium transporters, MGT2/MRS2-1 and MGT3/MRS2-5, are important for magnesium Cobalt accumulation Cs accumulation Ionomic accumulation QTLs partitioning within Arabidopsis thaliana mesophyll vacuoles under serpentine conditions. New Phytologist 190: 583-594. Morrissey J, Baxter I, Lee J, Li L, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML. 2009. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21: 3326-3338 QTL (Ler × Cvi; Ler × Payne K, Bowen H, Hammond J, Hampton C, Lynn J, Mead A, Swarup K, Bennett Col-0 and Nd × Col-0) MJ, White PJ, Broadley MR. 2004. Natural genetic variation in caesium (Cs) accumulation by Arabidopsis thaliana. New Phytologist 162: 535-548. Genome sequencing Atwell S, Huang Y, Vilhjálmsson B, Willems G, Horton M, Li Y, Meng D, Platt A, (SNPs) Tarone AM, Hu TT et al. 2010. Genome-wide association study of 107 phenotypes in a common set of Arabidopsis thaliana inbred lines. Nature 465: 627-631 QTL (Bay-0 × Sha, Col-4 Buescher E, Achberger T, Amusan I, Giannini A, Ochsenfeld C, Rus A, Lahner B, × Ler-0, and Cvi-0 × Ler- Hoekenga O, Yakubova E, Harper JF et al. 2010. Natural genetic variation in selected 2) populations of Arabidopsis thaliana is associated with ionomic differences. PLoS One 5: e11081. Metabolome QTL (Bay-0 × Sha) Rowe HC, Hansen BG, Halkier BA, Kliebenstein DJ. 2008. Biochemical networks and epistasis shape the Arabidopsis thaliana metabolome. Plant Cell 20: 1199-1216 Molybdenum sequestration QTL (Col-0 × Ler) Baxter I, Muthukumar B, Park HC, Buchner P, Lahner B, Danku J, Zhao K, Lee J, Hawkesford M, Guerinot ML et al. 2008. Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). PLoS Genetics 4: e1000004 Phosphate acquisition Quantification on 5 Narang RA, Bruene A, Altmann T. 2000. Analysis of phosphate acquisition efficiency in efficiency. natural accessions. different Arabidopsis accessions. Plant Physiology 124:1786-1799 Phytate and phosphate QTL Bentsink L, Yuan K, Koorneef M, Vreugdenhil D. 2003. The genetics of phytate and concentrations phosphate accumulation in seeds and leaves of Arabidopsis thaliana, using natural variation. Theoretical & Applied Genetics 106: 1234-1243. Potassium QTL (Cvi × Ler) Harada H, Leigh RA. 1996. Genetic mapping of natural variation in potassium concentrations in shoots of Arabidopsis thaliana. Journal of Experimental Botany 57: 953– 960. Selenate QTL (Ler × Col-0) Zhang L, Byrne PF, Pilon-Smits EA. 2006. Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytologist 170: 33-42. QTL (Ws × Col-0, Ler × Zhang L, Abdel-Ghany SE, Freeman JL, Ackley AR, Schiavon M, Pilon-Smits EAH. Col-0) 2006. Investigation of selenate tolerance mechanisms in Arabidopsis thaliana. Physiologia Sodium Sulphur QTL (Ts-1 × Plantarum 128: 212-223. Tsu-1) Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, Salt DE. 2006. Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genetics 2: e210. Natural accessions and Baxter I, Brazelton JN, Yu D, Huang YS, Lahner B, Yakubova E, Li Y, Bergelson J, root specific qPCR Borevitz JO, Nordborg M et al. 2010. A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS Genetics 6: e1001193. QTL (Bay-0 × Sha) Hesse H, Hoefgen R. 2006. On the way to understand biological complexity in plants: Snutrition as a case study for systems biology. Cellular & Molecular Biology Letters 11: 3755. Loudet O, Saliba-Colombani V, Camilleri C, Calenge F, Gaudon V, Koprivova A, North KA, Kopriva S, Daniel-Vedele D. 2007. Natural variation for sulfate content in Arabidopsis thaliana is highly controlled by APR2. Nature Genetics 39: 896–900. Tissue accumulation of multiple Comparative, tissue- Waters B, Grusak MA. 2008. Whole-plant mineral partitioning throughout the life cycle in elements (including Mg, Ca, Fe, specific ionomics (3 Arabidopsis thaliana ecotypes Columbia, Landsberg erecta, Cape Verde Islands, and the Zn, K, P) accessions) mutant line ysl1ysl3. New Phytologist 177: 389-405. Sulphate content QTL (Ler × Cvi; Ler × Waters B, Grusak MA. 2008. Quantitative trait locus mapping for seed mineral Col-0) concentrations in two Arabidopsis thaliana recombinant inbred populations. New Phytologist 179: 1033-1047. QTL (Antwerp-1 × Ler) Ghandilyan A, Barboza L, Tisne S, Grainer C, Reymond M, Koornneef M, Schat H, Aarts MGM. 2009a. Genetics analysis identifies quantitative trait loci controlling rosette mineral concentrations in Arabidopsis thaliana under drought. New Phytologist 184: 180192. QTL (Ler × Kond, Ler × Ghandilyan A, Ilk N, Hanhart C, Mbengue M, Barboza L, Schat H, Koornneef M, ElAn-1, Ler × Eri-1) Lithy M, Vreugdenhil D, Reymond M et al. 2009b. A strong effect of growth medium and organ type on the identification of QTLs for phytate and mineral concentrations in three Arabidopsis thaliana RIL populations. Journal of Experimental Botany 60: 1409-1425. Water, anion content nitrogen availability. Zinc Fast neutron mutagenised M2 populations and HT ionomics profiling and QTL (Bay-0 × Sha) Lahner B, Gong J, Mahmoudian M, Smith EL, Abid KB, Rogers EE, Guerinot ML, Harper JF, Ward JM et al. 2003. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nature Biotechnology 21: 1215-1221. Loudet O, Chaillou S, Krapp A, Daniel-Vedele F. 2003. Quantitative trait loci analysis of water and anion contents in interaction with nitrogen availability in Arabidopsis thaliana. Genetics 163: 711-722. Comparative, tissue- Richard O, Pineau C, Loubet S, Chalies C, Vile D, Marques L, Berthomieu P. 2011. specific ionomics (27 Diversity analysis of the response to Zn within the Arabidopsis thaliana species revealed a accessions) low contribution of Zn translocation to Zn tolerance and a new role for Zn in lateral root development. Plant, Cell & Environment. 34: 1065-1078. QTL – F2 population (T. Deniau AX, Pieper B, Ten Bookum WM, Lindhout P, Aarts MGM, Schat H. 2011. caerulescens varying in QTL analysis of cadmium and zinc accumulation in the heavy metal hyperaccumulator Cd and Zn) Thlaspi caerulescens. Theoretical & Applied Genetics 113: 907-920.