RECENZIE
advertisement

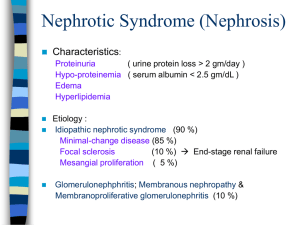
Nephrotic Syndrome in Children Author: Angela Ciuntu, Associate professor Pediatry No.1 Department SMPhU “NicolaeTestemiţanu” 1 Nephrotic syndrome is a common type of kidney disease seen in children. Historically, Roelans is credited with the first clinical description of nefrothic syndrome in the late fifteenth century, whereas Zuinger later provided a detailed description of the clinical course of the disease and its importance as a cause of chronic renal failure in the presteroid era. Nephrotic syndrome is characterized by massive proteinuria, hipoalbuminemia, and edema, although additional clinical features such as hyperlipidemia are also usually present. In the first few years of life, children with or this condition often show periorbital swelling with or without generalized edema. Nephrotic syndrome develops when the loss of protein in urine exceeds the rate of albumin synthesis in the liver, resulting in hypoalbuminemia and edema. Nephrotic syndrome may be caused by variety of glomerular and systemic diseases, but by far the most common type in childhood is idiopathic nephrotic syndrome. Before the introduction of antibiotics, corticosteroids, and other immunosuppressive therapies, nephrotic syndrome was associated with mortality as 67 %, usually following infections. Between 1967 and 1974m the International Study of Kidney Disease in Chidhood (ISKDC) enrolled 521 children age 12 weeks to 16 years with idiopathic nephritic syndrome in order to evaluate the histopathologic, clinical, and laboratory characteristics of nephritic syndrome in children. The renal biopsy studies demonstrated that about 80 % of children had either minimal change disease (MCD 76,4 %), focal and segmental glomerulosclerosis (FSGS 6,9 %), or mesangioproliferative glomerulonephritis (Mes PGN 2,3 %). Subsequently the ISKDC demonstrated that the response to corticosteroids was higly predictive of renal histology, with 93 % of children with MCD achieving complete remission following an 8-week course of prednisone. However, between 25 % and 50 % of children with Mes PGN or FSGS on biopsy also responded of prednisone. At the moment there is no diagnostic marker for children displaying with nephrotic syndrome that can be used as a predictor of steroid responsiveness or resistance. The 2 most important prognostic marker for children with nephrotic syndrome is their response to steroid treatement. Initial steroid treatement can be avoided only in patients with a family history of SRNS or in those who have a know gene mutation. DEFINITIONS TABLE 1. Definitions Used in Idiopathic Nephrotic Syndrome Classification Nephrotic syndrome Remission Initial responder Initial nonresponder/ steroid resistance Relapce Infrequent relapce Frequent relapce Steroid dependence Late nonresponder Definition Edema, proteinuria > 40 mg/m2/hr or protein/creatinine ratio > 0,2 g/mmol (> 2 g/g) or 50 mg/kg/day or 3-4 + on urine dipstick, hypoalbuminemia < 25 g/L (< 2,5 mg/100ml) Urinary protein excretion ≤ 4 mg/m2/hr or 0-trace of protein on urine dipstick or protein/creatinine ratio < 0,02 g/mmol (< 2 g/g) for 3 consecutive days Attainment of complete remission within initial 8 weeks of corticosteroid therapy Failure to achieve remission during initial 8 weeks of corticosteroid therapy Urinary protein > 40 mg/m2/hr or protein/creatinine ratio> 0,2 g/mmol (> 2 g/g) or 2 + protein or more on urine dipstick for 3 consecutive days One relapce within 6 months of initial response or one to three relapses in any 12- months period Two or more relapses within 6 months of initial response or four or more relapses in any 12- months period Two consecutive relapses during corticosteroid therapy or within 14 days of ceasing therapy Proteinuria for > 8 weeks following one or more remissions Nephrotic Syndrome: Diagnosis of nephritic syndrome requires the presence of edema, massive proteinuria (> 40 mg/m2/hr or a urine protein/creatinine ratio > 2,0 mg/mg), hypoalbuminemia (< 2,5 g/dl). Remission: Remission is characterized by a marked reduction in proteinuria (< 4 mg/m2/hr or urine albumin dipstick of to trace for 3 consecutive days) in association with resolution of edema and normalization of serum albumin to at least 3,5 g/dl. 3 Relapce: Relapce is defined as reccurence of massive proteinuria (> 40 mg/m2/hr, urine protein/creatinine ratio > 2,0 mg/mg, or urine albumin dipstick ≥ 2 + on 3 consecutive days), most often in association with recurrence of edema. Steroid-Sensitive Nephrotic Syndrome: Patients who enter remission in response to corticosteroid treatment alone are referred to as having steroid-sensitive nephrotic syndrome (SSNS). Steroid-Resistant Nephrotic Syndrome: Among pediatric nephrologists there are two definitions of Steroid-Resistant Nephrotic Syndrome (SRNS). The definitions introduced by the International Study of Kidney Disease in Children (ISKDC) and used by the Arbeitsgemeinschaft fȕr Pȁdiatrische Nephrologie (APN) is widely accepted as follows: No urinary remission within 4 weeks of prednisone therapy 60mg/m2/day. The other definition, employed by the Society of French Speaking Pediatric Nephrologists, states: No urinary remission following 4 weeks of prednisone 60mg/m2/day followed by three intravenous pulses of methylprednisolone. Steroid-Dependent Nephrotic Syndrome: some patients respond to initial corticosteroid treatment by entering complete remission but develop a relapse either while still receiving steroids or within 2 weeks discontinuation of treatment following a steroid taper. Such patients typically require continued low-dose treatment with steroids to prevent development of relapse, and are therefore reffered to as having steroid-dependent nephrotic syndrome (SDNS). Frequent Relapsing Nephrotic Syndrome: patients in this goup enter complete remission in response to steroids. Thery remain in remission for several weeks following discontinuation of treatment but develop frequent relapses. If relapses occur 4 or more times in any 12-months period, these patients are referred to as having frequent relapsing nephrotic syndrome (FRNS). Both SDNS and FRNS patients are at increased risk of developing complications of nephrotic syndrome and complications from frequent use of steroids and other immunosuppressive agents. 4 EPIDEMIOLOGY The incidence of SRNs varies throughout the world. In Europe, the United States, and Australia, the overall incidence of idiopathic nephrotic syndrome is 1 to 3 per 100.000 children below age 16, with a cumulative prevalence of 16 per 100.000 children. The incidence is higher in Asian, African American, and Arab children. In Asian children residing in northern England, the overall rates are 7,4 (95 % confidence intervals [CI] 5,3-9,5) for South Asian children compared with 1,6 (95 % CI 1,3-1,8) per 100.000 children per year for non- South Asian children with 88 % responsive to corticosteroids. In Libya an incidence of 11,6 per 100.000 children was reported, with 98 % responsive to corticosteroids. In African American children, rates of 2,8 to 3,6 per 100.000 per year have been reported compared with 1,8 to 2,3 in Caucasian children. SSNS is more common in boys than in girls, with a male/femaleratio of around 2:1 and a peak incidence between 1 and 4 years. There is a decreasing trend with increasing age in the proportion with SSNS (Table 2). SSNS is lesscommon in African and African American children. In South Africa only 7,2 % of 236 African children had SSNS compared with 62 % of 286 African children. In the past 2 decades the proportion of children with idiopathic nephrotic syndrome who respond to corticosteroids appears to be falling when compared with 1978 ISKDC data. Among 159 Canadian children age 6 months to 19 years, the proportion with SSNS fell from 81 % between 1985 and 1993 to 65 % between 1993 and 2002; this was accompanied by an increase in biopsy-documented FSGS have been reported from South Africa and the United States. Age also correlates with both the frequeny of presention and the biopsy findings associated eith nephrotic syndrome. The most common age for presentation is 2 years and 70 % to 80 % of cases occur in children younger than. To somme extent age also predicts the histologic lesion associated with nephrotic syndrome. Children diagnosed bef age 6 represented 79,6 % of those with FSGS and only 2,6 % of those 5 membranoproliferative glomerulonephritis (MPGN). With these data were analyzed on the basis of renal histology, median ages at presentation were found to be 3 years excluding the first year of life, these data combined su that the likelihood of having MCNS decreases with increases age, whereas the likelihood of having the less favorable diagnosis of FSGS or MPGN increases. The histologic lesion associated with nephrotic syndrome has important ramifications for the likelihood of response steroid treatement. Although almost 80 % of children diagnosed with nephrotic syndrome in a multicenter International Study of Kidney Diseases in Children (ISKDC) entered remission following an initial 8-week course prednisone, when these children were analyzed based histology, steroid responsiveness was found in 93 % of with MCNS compared with only 30 % of those with FSGS and 7 % of those with MPGN. In addition to histology response to steroids also vries with geographic location ethnicity. Whereas 80 % of children in western countries be steroid responsive, studies from South Africa, Nigeria more recently Ghana show that only 9 % to 50 % of children with nephrotic syndrome are steroid responsive. ETIOLOGY The etiology of nephrotic syndrome is also age depend. Most cases appearing in the first 3 months of life are re as congenital nephrotic syndrome (CNS) and are genetic diseases. Although there has been no systematic of the etiology of nephrotic syndrome presenting in the the first year of life (3 to 12 months), there are data sugg that up to 40% of cases during this time may also be during genetic causes. Beyond the first year of life and in the decade, most cases are due to primary or idiopathic nephrotic syndrome cases increases beyond the first 10 years of life. ETIOLOGY AND PATHOGENESIS A T-Cell Disease In 1974 a series of clinical observations led Dr. Shaloub to propose that SRNS was due to an abnormality in T-cell function. Nephrotic syndrome had been observed in patients with Hodgkins lymphoma and cases of thymoma. The desease was noted to 6 remit in children who had measles, which led some to propose using measles as a therapeutic strategy. A major effect of the measles virus is that it inhibits cell-mediated immunity, thereby shutting down T-cell function. Furthermore, the response of nephrotic syndrome to T-cell suppressive agents such as steroids or calcineurin inhibitors also supported their role in nephrotic syndrome. These features all suggest that lymphocytes are key cells in SSNS. A Circulating Factor MCD appears to exist in a spectrum with FSGS. A proportion of children with MCD on clinical and histologic grounds develop FSGS. In both there appears to be a circulating factor, with FSGS children being less responsive to therapeutic agents for various reasons. Within this group is a subset of children in which the disease resides in structural changes in the glomeruli with genetic mutations in key glomerular slit process proteins, including nephrin, podocin, Actinin 4, and WT-1. These are described elsewhere but in brief are associated with no response to steroids and progression to endstage renal failure, and do not show evidence of a circulating factor as demostrated by rapid recurrence of disease in a transplanted kidney. The timing of response with the return to normal function taking days to weeks is also supportive of slow podocyte recovery from an injurious cytokine. The higher rates of recurrence in children with FSGS receiving living-related kidneys suggests that there may be a degree of HLA restriction of response, which is also supported by HLA-linkage studies showing that increased incidence of desease is tied to certain alleles such as HLA B8, B13, DWQ2, DQB10301, and DR7. Over the years vartous growth factors and cytokines have been proposed as pathogenic in SSNS. The initial identification of the vascular permiability factor (VPF), now called vascular endothelial growth factor (VEGF), was thought to have identified the key protein leading to nephrotic syndrome. However, identification of this protein in norm urine delayed further investigation of its role. More recen it has been noted to be increased in urine during relapses nephrotic syndrome though circulating levels are unchange suggesting that VEGF levels reflect the concomitant 7 proteinuria. Recent tissue-restricted knockouts of VEGF in mice restricted to podocytes have demonstrated a key role for loc VEGF in maintaining glomerular endothelial integrity and again have reinforced its importance, though perhaps more locally, in maintaining permeability. Soluble immun response suppressor (SIRS) was also identified as a cytokine in patients with SRNS, but again the inability to consistent characterize this protein despite many mechanistic observations led to its exclusion as the likely factor. Other circulating factors have been proposed, and development of a functional assay of glomerular permeability by Dr. Savin in the late 1990s identified a proteinuric factor that was small, highly glycosylated, and hydrophobic. The appeared likely to allow fractionation of nephrotic sera, wh would allow identification of the factor. Other, observation that protein A coluns could remove the nephrotic factor posttransplant also seemed to point to identifying features. PATHOGENESIS The central abnormality in all cases of nephrotic syndrome is the development of massive proteinuria. Although the molecular basis for this is still speculative, there is evidence in the literature that nephrotic syndrome may be a consequence of a primary glomerular defect, circulating factors, or an immunological abnormality. Primary Glomerular Defect One of the most important functions of the kidney is the filtration of blood by glomeruli, which allows excretion of fluid and waste products while retaining the majority of blood proteins and all blood cells within the vasculature. This process of filtration is made possible by the glomerular filtration barrier, which is made up of specialized fenestrated endothelial cells, the glomerular basement membrane (GBM). Neighboring podocyte foot processes are connected to each other by networks of specialized cell-cell junctions known as slit diaphragms. In addition, the GBM has an abundant supply of negatively charged heparin sulfate proteoglycan, resulting in negatively charged molecules being relatively more restricted from passage than 8 positively charged molecules of the same size. In health, molecules greater than 42 A in diameter, or more than 200 kDa, are unable to cross the filtration barrier. This restriction depends largely on the structural integrity of the podocyte foot processes and slit diaphragms, as well as the GBM charge. In nephrotic drome there of negative charge of the GBM. O morphologic changes in podocytes that occur during development of nephrotic syndrome include sweiling, retraction, effacement (spreading) of the podocyte distal foot proces vacuole formation, occurrence of occluding junctions, placement of slit diaphragms, and detachment of podocyte from the GBM. The importance of podocyte and slit diaphragm struc to the pathogenesis of nephrotic syndrome is further forced by recent observations in humans and axperiment animals that mutations in genes encoding some of the diaphragm proteins or their transcription factors can SRNS and/or FSGS. Table 2. Etiologies of Nephrotic Syndrome (Beyond 3 Months of Age) Idiopathic Genetic Autosomal recessive FSGS due to mutation in gene encoding podocin (NPHS2) Autosomal dominant diffuse mesangial sclerosis (DMS) due to mutation in gene encoding WTI Autosomal dominant FSGS due to mutation in gene encoding αactinin 4 Autosomal dominant FSGS due to mutation in gene encoding CD2-associated protein (CD2AP) Minimal change nephrotic syndrome (MCNS) Focal segmental glomerulosclerosis (FSGS) Mesangial proliferative glomerulonephritis Membranoproliferative glomerulonephritis (MPGN) Membranous nephropathy (MN) Ig M nephropathy Ciq nephropathy Autosomal dominant FSGS due to mutation in gene encoding transient receptor potential cation chan 6 (TRPC6) Infections Hepatitis B and C 9 HIV Malaria Schistosomiasis Filariasis Systemic disease Henoch-Schonlein purpura Systemic lupus erythematosus Diabetes mellitus Sarcoidosis Metabolic disease Fabry's disease Glutaric acidemia Glycogen storage disease Mitochondrial cytopathies Hematologic Leukemia and oncologic Lymphoma (Hodgkin's most likely can lead minimal change) disease Sickle cell disease Drugs Nonsteroidal antiinflammatory drugs (NSAIDs) Gold Penicillamine Angiotensin converting enzyme inhibitors (ACEIs) Pamidronate Interferon Mercury Heroin Lithium Others Bee stings (MCNS) Food allergies Obesity (usually with FSGS) Oligomeganephronia Pregnancy 10 Circulating factors There are experimental data to support the existence of soluble mediators that may alter capillary wall permeability in nephrotic syndrome. Evidence for this includes development of nephrotic syndrome in newborn babies born to mothers with nephrotic syndrome who apparently transferred a soluble factor to their fetuses in utero, marked reduction of proteinuria following treatement with protein A immunoadsorption in various types of primary nephrotic syndrome, recurrence of FSGS in transplanted kidneys in patients with induced by treatement with protein A immunoadsorption due to presumed removal of circulating factors, and induction of enhanced glomerular permeability in experimental animals injected with serum from patient with FSGS recurrence in transplanted kidneys. Furthertmore, inhibitors of glmerular permeability have also been isolated from the serum of children with FSGS and identified as components of apolipoproteins, suggesting that an imbalance between serum permeability factors and permeability inhibitors may have a pathogenic role in FSGS. Immunological Abnormality The theory that nephrotic syndrome may be due to dysregulation of the immune system has existed for more than 30 years. There are numerous reports of abnormalities of both the humoral and cellular immune responses during relapse of nephrotic syndrome. However, the idea that nephrotic syndrome may be due to dysregualtion of T lymphocyte function was first proposed by Shalhoub and his colleagues. Evidence for this includes responsiveness of most forms of primary nephrotic syndrome to corticosteroids, alkylating agents, calcineurin inhibitors, and mycophenolate mofetil, all of which are known inhibitors of T lymphocyte function, induction of remission of nephrotic syndrome following infections with measles and malaria, diseases known to depress cell-mediated immunity, and identification of MCNS as a paraneoplastic manifestation of Hodgkin's diseases and other lymphoreticular malignancies. Other reports have also suggested an important role of the cell-mediated immunyti during relapses of MCNS alterations in t cell subsets 11 during relapses, and increased cell surface expression of IL-2 receptors on T cells, reflective of T cell activation. In addition, numerous cytokines, released in part by T lymphocytes, have been reportedto be variably altered during nephrotic syndrome. It should be noted, however, that despite numerous reports, none of these cytokines has proven to be both present in the majotity of cases of MCNS and able to induce significant proteinuria in experimental animals. 12 Massive proteinuria Hypoalbuminemia Reduced intravascular oncotic pressure Fluid shift to extravascular compartment Intravascular volume depletion Inhibition of ANP Activation of SNS Activation of RAAS Release of ADH Intravascular volume depletion Edema Continued salt and water intake Fig. 1. Underfill hypothesis of edema formation in nephrotic syndrome. 13 CLINICAL FEATURES AND DIAGNOSIS History and Physical Examination In a child with periorbital or generalized edema, the primary care physician can quickly make this diagnosis by documenting significant proteinuria with more than 2+ albumin on urine dipstick or a spot urine protein/creatinine ratio greater than 2 mg/mg and serum albumin of less than 2,5 g/dl. In addition, a careful history should exclude possible complications and identify children with atypical presentations that might reflect other serious systemic illnesses. It should include an evaluation of any abdominal distension, which is usually due to ascites and sometimes tension may be accompanied by abdominal discomfort, persistent abdominal pain may be abdominal discomfort, persistent abdominal pain may be due to primary bacterial peritonitis (a potentially life-threatening complication), gut edema, or relative gut ischemiadue to hypoperfusion secondary to intravascular volume depletion. Other causes of an acute abdomen should also be considered. Regarding physical examination, blood pressure should be carefully determined in nephrotic children, it can be either low (due to intravascular volume depletion) or elevated (due to neurohumoral responses to hypovolemia, intrinsic renal causes, or occasionally renal vein thrombosis). Hypertension has been reported in up to 21 % of children 6 years and under with biopsy-confirmed MCNS, and may be present in up to 50 % of children with other histologic types. Laboratory Evaluation Diagnosis of nephrotic syndrome is confirmed by the triad of generalized edema, proteinuria, albuminuria (> 2+ on dipstick or urine protein/creatinine ratio (>2mg/mg), and hypoalbuminemia (serum albumin < 2,5 g/dl), although hypercholesterolemia is also commonly present. In patients with a typical presentation, serum studies should include an evaluation of complete blood count, electrolytes, blood urea nitrogen (BUN), creatinine, and albumin levels. For patients at an older age at presentation or with atypical presentation, additional serum studies to exclude secondary causes of nephrotic syndrome should include C3 and C4 complement levels; antinuclear antibody (ANA) and possibly anti-double-stranded DNA; HIV antibody; hepatitis A, B, and C serologies, and consideration of other viral serologies such as HIV antibodies. Because immunosuppressive therapy is the mainstay of treatment for most cases of childhood nephrotic syndrome, many pediatric nephrologists recommend placing a PPD (purified protein derivative) test to screen for occult tuberculosis before instituting immunosuppression. Renal ultrasound does not usually have a role in the evaluation of childhood nephrotic syndrome. However, in thesetting of a nephrotic child who develops gross hematuria, thrombocytopenia, or unexplained persistent hypertension, renal ultrasound should be considered to exclude possible development of renal vein thrombosis. 14 Renal Biopsy More than 80 % of children with idiopathic nephrotic syndrome will respond to steroid therapy by entering complet remission. Based on this statistic, an initial trial of 4 weeks of high-dose daily steroid therapyis usually prescribe in children under 10 before considering renal biopsy. Is general, renal biopsy is indicated only in the setting of atypical features such as age at onset (less than 1 year or m than 10), SDNS or SRNS, gross or persistent microscopic hematuria or presence of red cell casts, abnormal serologies, or significant persistent renal failure. TREATMENT OF NEPHROTIC SYNDROME Specific Therapy The initial treatement for new-onset nephrotic syndrome erally includes 60mg/m2/day (maximum 80 mg/d) of prednisone for 4 to 8 weeks, followed by 40mg/m2 every e day for 4 to 8 weeks, and then a gradual taper until discontinued. In patients, FRNS and SDNS, alternative agentswith potential steroid sparing effects are often used, including cyclophosphamide, levamisole, cyclosporine, tacrolimus, and mycophen mofetil. In patients with SRNS, however, the most monly used agents include cyclosporine, tacrolimus, hi dose intravenous methylprednisolone, and mycopheno mofetil (MMF), although the efficacy of almost all tha agents is lower in these patients compared with FRNS SDNS patients. General Management Edema Patients with nephrotic syndrome have increased total b fluid and sodium during active disease. General measure control edema include salt restriction, moderate fluid restriction, and judicious use of diuretics. Dietary recommendation include maintenance of protein intake at approximately to 140 % of the RDA for age, as well as avoidance of satural fats that can worsen hyperlipidemia. Because the intravascular volume status in children nephrotic syndrome is typically low, diuretics should ally be used only when significant intravascular depi has been either excluded or corrected. Typically corre of intravascular depletion can be achieved by ini intravenous 25 % albumin at 1-2 g/kg/d either as a contin infusion or divided q 6-8 hours. Albumin treatement should continue for 4 to 6 hours before initial administration of diuretics to minimize the risk of worsening any intravascular volum depletion that may be present. The most commonly used diuretic in this setting is the loop diuretic furosemide. It acts by inhibiting the sodium-potassium-2 chloride transporter in the thick ascending limb of the loop of Henle. During nephrotic syndrome, however, several factors may impair its efficacy. Because furosemide is higly protein bound, hypoalbuminemia may result in reduced delivery of albumin- bound furosemide to the proximal tubular cells for secretion into the tubular lumen. Hypoalbuminemia also causes an increased 15 volume of distribution of furosemide due to diffusion of the free drug into the expanded interstitial compartment. Measures to overcome resistance to furosemide include increased dose, coadministration with albumin, and coadministration with distal tubular diuretics. Doses ranging from 200 % to 300 % of normal can often achieve the desired clinical effects, although high doses in the presence of significant renal impairment may increase the risk for ototoxicity, which has been shown to be related to the peak levels. Clinically effective dosing strategies for intravenous furosemide in nephrotic children with normal renal function typically range from 0,5-1 mg/kg q 6-12 hours, although reports in children with cardiac disease have shown that continuous infusion of furosemide results in a more efficient diuresis compared with intermittent administration. When diuretics are used, physicians should watch closely for common and serious side effects of 3 agents, which include increased risk of thrombosis, electrolyte disturbances such as hypokalemia and metabolic alkalosis, pypercalciuria and nephrocalcinosis, and ototoxicity. Hyperlipidemia Hyperlipidemia is commonly found in children with nephrotic syndrome. The characteistic lipid profile includes elevations in total plasma cholesterol, very-lowdensity lipoprotein (VLDL), and low-density lipoprotein (LDL) cholesterol, triglyceride, and lipoprotein A, as well as variable alterations (more typically decreased) in high-density lipoprotein (HDL) cholesterol. Although hyperlipidemia in children with SSNS is often transiet and usually returns to normal after remission, children with SRNS refractory to therapy often have sustained hyperlipidemia. The potential usefulness of hydroxymethylglutaryl CoA (HMG CoA) reductase inhibitors (statins) in children with SRNS has been reported in a few uncontrolled trials. Antiproteinuric Agents Angiotensin converting enzyme inhibitors (ACEIs) are increasingly being used in the management of persistent proteinuria and control of hypertension in children with SRNS or SDNS. The antiproteinuric effects of ACEIs are due to thein ability to reduce glomerular capillary plasma flow rate, decrease transcapillary hydraulic pressure, and alter the permselectivity of the glomerular filtration barrier. COMPLICATIONS Infection Thromboembolism Cardiovascular Disease Respiratory Distress Bone Disease 16 Acute renal Failure Other Complications TABLE 3. Complications of Nephrotic Syndrome Infectious Peritonitis Cellulitis Disseminated Varicella Infection Cardiovascular Hypertension Hyperlipidemia Coronary artery disease Respiratory Pleural effusion Pulmonary embolism Hematologic Venous (more common) or arterial (less common) thrombosis Anemia Gastrointestinal Intussusception Renal Acute renal failure Renal vein thrombosis Endocrinologic Reduced bone mineral density Hypothyroidism, clinical and subclinical (more common in CNS) Neurologic Cerebral venous thrombosis Treatement-related General Infection, hypertension Steroids Growth impairment, reduced bone density, posterior capsular cataracts, avascular necrosis of femoral head Alkylating agents Hemorrhagic cystitis, dose-related oligospermia and premature ovarian failure, increased risk of malignancy Calcineurin inhibitors Gingival hyperplasia, hirsutism, hyperkalemia, encephalopathy Mycophenolate mofetil (MMF) Nausea, vomiting, diarrhea, constipation, dose-related leukopenia, headache PROGNOSIS 17 The single most important prognostic factor for maintenance of long-term normal renal function in nephrotic syndrome is the patient's initial response to corticosteroids. Although children who enter complete remission during an 8-week initial course of oral corticosteroids have an excellent prognosis, the prognosis for those who fail to enter remission is more guarded. Overall, close to 80 % of newly diagnosed children treated with corticosteroids will achieve complete remission. 18